DOI:
https://doi.org/10.14483/22487638.18970Published:
2023-11-03Issue:
Vol. 27 No. 77 (2023): Julio - SeptiembreSection:
ReviewRelación entre aluminio y la enfermedad de Alzheimer: Revisión
Relationship between aluminum and Alzheimer's Disease: Review
Keywords:
contaminated food, dementia, health, heavy metal, migration, water (en).Keywords:
alimentos contaminados, demencia, salud, metales pesados, migración, agua (es).Downloads
References
Adani, G., Filippini, T., Garuti, C., Malavolti, M., Vinceti, G., Zamboni, G., Tondelli, M., Galli, C., Costa, M., Vinceti, M. & Chiari, A. (2020). Environmental risk factors for early-onset Alzheimer’s dementia and frontotemporal dementia: A case-control study in northern Italy. International Journal of Environmental Research and Public Health, 17(21), 7941. https://doi.org/10.3390/ijerph17217941 DOI: https://doi.org/10.3390/ijerph17217941
Assal, F. (2019). History of Dementia. A History of Neuropsychology, 44, 118–126. https://doi.org/10.1159/000494959 DOI: https://doi.org/10.1159/000494959
Al Juhaiman, L. (2015). Estimating Aluminum Leaching into Meat Baked with Aluminum Foil Using Gravimetric and UV-Vis Spectrophotometric Method. Food and Nutrition Sciences, 6, 538-545. https://doi.org/10.4236/fns.2015.65056 DOI: https://doi.org/10.4236/fns.2015.65056
Al Zubaidy, E. A., Mohammad, F. S., & Bassioni, G. (2011). Effect of pH, salinity and temperature on aluminum cookware leaching during food preparation. Int. J. Electrochem. Sci, 6(12), 6424-6441 DOI: https://doi.org/10.1016/S1452-3981(23)19691-X
Alzheimer’s Association (2023). 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement, 19(4). https://doi.org/10.1002/alz.1301
Arcila, H. R., & Peralta, J. J. (2015). Agentes naturales como alternativa para el tratamiento del agua. Revista Facultad de Ciencias Básicas, 11(2), 136-153. https://doi.org/10.18359/rfcb.1303 DOI: https://doi.org/10.18359/rfcb.1303
Ballard, C., Mobley, W., Hardy, J., Williams, G., & Corbett, A. (2016). Dementia in Down’s syndrome. The Lancet Neurology, 15(6), 622-636. https://doi.org/10.1016/S1474-442200063-6 DOI: https://doi.org/10.1016/S1474-4422(16)00063-6
Barthel, H. (2020). First tau PET tracer approved: toward accurate in vivo diagnosis of alzheimer disease. Journal of Nuclear Medicine, 61(10), 1409-1410. https://doi.org/10.2967/jnumed.120.252411 DOI: https://doi.org/10.2967/jnumed.120.252411
Bassioni, G., Mohammed, F. S., Al Zubaidy, E., y Kobrsi, I. (2012). Risk assessment of using aluminum foil in food preparation. Int. J. Electrochem. Sci, 7(5), 4498-4509. DOI: https://doi.org/10.1016/S1452-3981(23)19556-3
Bejarano, J. J. & Suárez, L. M. (2015). Algunos peligros químicos y nutricionales del consumo de los alimentos de venta en espacios públicos. Revista de la Universidad Industrial de Santander. Salud, 47(3), 349-360. DOI: https://doi.org/10.18273/revsal.v47n3-2015011
Bichu, S., Tilve, P., Kakde, P., Jain, P., Khurana, S., Ukirade, V., Jawandhiya, P., Dixit, A., Bhasin, N., Billa, V., Kumar, R., Kothari, J. & Kothari, J. (2019). Relationship between the Use of Aluminium Utensils for Cooking Meals and Chronic Aluminium Toxicity in Patients on Maintenance Hemodialysis: A Case Control Study. The Journal of the Association of Physi cians of India, 67(4), 52-56.
Bocca, B., Forte, G., Oggiano, R., Clemente, S., Asara, Y., Peruzzu, A., Farace C., Pala S., Fois A. G., Pirina P., & Madeddu, R. (2015). Level of neurotoxic metals in amyotrophic lateral sclerosis: a population-based case–control study. Journal of the Neurological Sciences, 359(1-2), 11-17. https://doi.org/10.1016/j.jns.2015.10.023 DOI: https://doi.org/10.1016/j.jns.2015.10.023
Bondy, S. C. (2016). Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology, 52, 222-229. https://doi.org/10.1016/j.neuro.2015.12.002 DOI: https://doi.org/10.1016/j.neuro.2015.12.002
Bortoli, P. M., Alves, C., Costa, E., Vanin, A. P., Sofiatti, J. R., Siqueira, D. P., Dallago, R.M., Trei chel, H., Delise, G., Vargas, L.P. & Kaizer, R. R. (2018). Ilex paraguariensis: Potential anti oxidant on aluminium toxicity, in an experimental model of Alzheimer’s disease. Journal of inorganic biochemistry, 181, 104-110. https://doi.org/10.1016/j.jinorgbio.2017.11.001 DOI: https://doi.org/10.1016/j.jinorgbio.2017.11.001
Bratakos, S. M., Lazou, A. E., Bratakos, M. S., & Lazos, E. S. (2012). Aluminium in food and daily dietary intake estimate in Greece. Food Additives and Contaminants: Part B, 5(1), 33-44. https://doi.org/10.1080/19393210.2012.656289 DOI: https://doi.org/10.1080/19393210.2012.656289
Dos Santos, C. C. M., Nauar, A. R., Ferreira, J. A., da Silva Montes, C., Adolfo, F. R., Leal, G., Reis, G.M., Lapinsky, J., de Carvalho, L. M. & Amado, L. L. (2023). Multiple anthro pogenic influences in the Pará River (Amazonia, Brazil): A spatial-temporal ecotoxico logical monitoring in abiotic and biotic compartments. Chemosphere, 323, 138090. https://doi.org/10.1016/j.chemosphere.2023.138090 DOI: https://doi.org/10.1016/j.chemosphere.2023.138090
Chen, R. F., Shen, R. F., Gu, P., Wang, H. Y., & Xu, X. H. (2008). Investigation of Aluminum Tolerant Species in Acid Soils of South China. Communications in Soil Science and Plant Analysis, 39(9-10), 1493-1506. https://doi.org/10.1080/00103620802006610 DOI: https://doi.org/10.1080/00103620802006610
Chen, R. Y., Qiao, Q. J., Diao, C. X., Hu, J. M., Yan, S. W., & Huang, H. P. (2017). [Investigation on the aluminum content in wheat and wheat flour] (in Chinese). Chinese Journal of Health Laboratory Technology, 27(19), 2864–2866.
Deng, G., He, Y., Lu, L., Wang, F., & Hu, S. (2021). Comparison between Fly Ash and Slag Slurry in Various Alkaline Environments: Dissolution, Migration, and Coordination State of Aluminum. ACS Sustainable Chemistry y Engineering, 9(36), 12109-12119. https://doi.org/10.1021/acssuschemeng.1c03434 DOI: https://doi.org/10.1021/acssuschemeng.1c03434
Diamond, J. (2008). A report on Alzheimer’s disease and current research. Alzheimer Society of Ca nada. http://brainxchange.ca/Public/Files/Events/National/ADandCurrentResearch_DrJackDiamond.aspx [Citado 29 de junio de 2021].
De Silva J, Tuwei G, Zhao FJ (2016) Environmental factors influencing aluminium accumu lation in tea (Camellia sinensis L.). Plant Soil 400(1):223–230. https://doi.org/10.1007/s11104-015-2729-5 DOI: https://doi.org/10.1007/s11104-015-2729-5
Ejovwokoghene, I. J., & Philipa, U. O. (2020). Risk of Consuming Aluminum in Barbeque Cat fish Prepared with Aluminum Foil. Statement of Purpose and Objective, 21.
Ekong, M. B., Ekpo, M. M., Akpanyung, E. O., & Nwaokonko, D. U. (2017). Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degenera tion. Metabolic brain disease, 32(5), 1437-1447. https://doi.org/10.1007/s11011-017-0011-7 DOI: https://doi.org/10.1007/s11011-017-0011-7
Elufioye, T. O., Chinaka, C. G., & Oyedeji, A. O. (2019). Antioxidant and anticholinesterase ac tivities of Macrosphyra longistyla (DC) Hiern relevant in the management of Alzheimer’s Disease. Antioxidants, 8(9), 400. https://doi.org/10.3390/antiox8090400 DOI: https://doi.org/10.3390/antiox8090400
Ertl, K., & Goessler, W. (2018). Aluminium in foodstuff and the influence of aluminium foil used for food preparation or short time storage. Food Additives & Contaminants: Part B,11(2), 153-159. https://doi.org/10.1080/19393210.2018.1442881 DOI: https://doi.org/10.1080/19393210.2018.1442881
Exley, C., & Esiri, M. M. (2006). Severe cerebral congophilic angiopathy coincident with in creased brain aluminium in a resident of Camelford, Cornwall, UK. Journal of Neurology,Neurosurgery y Psychiatry, 77(7), 877-879. http://dx.doi.org/10.1136/jnnp.2005.086553 DOI: https://doi.org/10.1136/jnnp.2005.086553
Exley, C., y Vickers, T. (2014). Elevated brain aluminium and early onset Alzheimer’s disease in an individual occupationally exposed to aluminium: a case report. Journal of medical case reports, 8(1), 1-3. https://doi.org/10.1186/1752-1947-8-41 DOI: https://doi.org/10.1186/1752-1947-8-41
Exley, C. (2017). Aluminum should now be considered a primary etiological factor in Alzhei mer’s disease. Journal of Alzheimer’s disease reports, 1(1), 23-25. http://dx.doi.org/10.3233/ADR-170010 DOI: https://doi.org/10.3233/ADR-170010
FAO/WHO Expert Committee on Food Additives. Meeting, and World Health Organiza tion. (2007). Evaluation of certain food additives and contaminants: sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives (Vol. 68). World Health Or ganization. https://apps.who.int/iris/bitstream/handle/10665/43870/9789241209472_eng.pdf?sequence=1&isAllowed=y [Cited on August 4th of 2021].
FAO/WHO. (2021). Codex Alimentarius: general standard for food additives. Codex Ali mentarius: general standard for food additive; Codex Stan 192-1995; FAO/WHO: Geneva, Switzerland. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B192-1995%252FCXS_192e.pdf. [Cited on February 12th of 2021].
Fermo, P., Soddu, G., Miani, A., & Comite, V. (2020). Quantification of the aluminum content leached into foods baked using aluminum foil. International Journal of Environmental Research and Public Health, 17(22), 8357. https://doi.org/10.3390/ijerph17228357 DOI: https://doi.org/10.3390/ijerph17228357
García, M. C., García, C. A., & de Plaza, J. S. (2016). Estudio exploratorio del tratamiento de agua de lavado de tintas por método de electrocoagulación/electroflotación. Revista Tecnura, 20(47), 107-117. http://doi.org/10.14483/udistrital.jour.tecnura.2016.1.a09 DOI: https://doi.org/10.14483/udistrital.jour.tecnura.2016.1.a09
Gebhardt, B., Sperl, R., Carle, R., & Müller-Maatsch, J. (2020). Assessing the sustainability of natural and artificial food colorants. Journal of Cleaner Production, 260, 120884. https://doi.org/10.1016/j.jclepro.2020.120884 DOI: https://doi.org/10.1016/j.jclepro.2020.120884
Guo, J., Peng, S., Tian, M., Wang, L., Chen, B., Wu, M., & He, G. (2015). Dietary exposure to alu minium from wheat flour and puffed products of residents in Shanghai, China. Food Ad ditives & Contaminants: Part A, 32(12), 2018-2026. https://doi.org/10.1080/19440049.2015.1099078 DOI: https://doi.org/10.1080/19440049.2015.1099078
Hafez, H. H., Abd El-Salam, A. M., & Hamed, G. H. (2018). Studies on the effect of aluminum, aluminum foil and silicon baked cups on aluminum and silicon migration in cakes. Egy ptian Journal of Agricultural Research, 96(2), 565-574. https://doi.org/10.21608/ejar.2018.135752 DOI: https://doi.org/10.21608/ejar.2018.135752
Hardisson, A., Revert, C., Gonzales-Weler, D., & Rubio, C. (2017). Aluminium exposure th rough the diet. Food Sci. Nutr, 3, 1-10. https://doi.org/10.24966/FSN-0176/100019 DOI: https://doi.org/10.24966/FSN-1076/100020
Hippius, H., & Neundörfer, G. (2003). The discovery of Alzheimer’s disease. Dialogues in clini cal neuroscience, 5(1), 101-108. https://doi.org/10.31887/DCNS.2003.5.1/hhippius DOI: https://doi.org/10.31887/DCNS.2003.5.1/hhippius
Hu, X. F., Chen, F. S., Wine, M. L., & Fang, X. M. (2017). Increasing acidity of rain in subtropical tea plantation alters aluminum and nutrient distributions at the root-soil interface and in plant tissues. Plant and Soil, 417, 261-274. https://doi.org/10.1007/s11104-017-3256-3 DOI: https://doi.org/10.1007/s11104-017-3256-3
International-Aluminium. Primary Aluminium Production Date of Issue: 20 march 2023. https://international-aluminium.org/statistics/primary-aluminium-production/ [Citedon March 23rd of 2023].
Inan-Eroglu, E., Gulec, A. & Ayaz, A. (2019): Effects of different pH, temperature and foils on aluminum leaching from baked fish by ICP-MS. Czech J. Food Sci., 37, 165–172. https://doi.org/10.17221/85/2018-CJFS DOI: https://doi.org/10.17221/85/2018-CJFS
Iscuissati, I. P., Galazzi, R. M., Miró, M., & Arruda, M. A. Z. (2021). Evaluation of the aluminum migration from metallic seals to coffee beverage after using a high-pressure coffee pod machine. Journal of Food Composition and Analysis, 104, 104131. https://doi.org/10.1016/j.jfca.2021.104131 DOI: https://doi.org/10.1016/j.jfca.2021.104131
Jabeen, S., Ali, B., Ali Khan, M., Bilal Khan, M., & Adnan Hasan, S. (2016). Aluminum intoxica tion through leaching in food preparation. Alexandria Science Exchange Journal, 37, 618-626. https://doi.org/10.21608/asejaiqjsae.2016.2539 DOI: https://doi.org/10.21608/asejaiqjsae.2016.2539
Linhart, C., Talasz, H., Morandi, E. M., Exley, C., Lindner, H. H., Taucher, S., Egle, D., Hubalek, M., Concin, N., & Ulmer, H. (2017). Use of underarm cosmetic products in relation to risk of breast cancer: a case-control study. EBioMedicine, 21, 79-85. https://doi.org/10.1016/j.ebiom.2017.06.005 DOI: https://doi.org/10.1016/j.ebiom.2017.06.005
Kabir, M. T., Uddin, M. S., Zaman, S., Begum, Y., Ashraf, G. M., Bin-Jumah, M. N., Bungau S.G., Mousa S. A., & Mohamed M. Abdel-Daim & Abdel-Daim, M. M. (2021). Molecular mecha nisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Molecular neurobiology,58, 1-20. http://dx.doi.org/10.1007/s12035-020-02096-w DOI: https://doi.org/10.1007/s12035-020-02096-w
Kjaergaard, A. D., Johannesen, B. R., Sørensen, H. T., Henderson, V. W., & Christiansen, C. F. (2021). Kidney disease and risk of dementia: a Danish nationwide cohort study. BMJ open,11(10), e052652. http://dx.doi.org/10.1136/bmjopen-2021-052652 DOI: https://doi.org/10.1136/bmjopen-2021-052652
McLachlan, D. R., Alexandrov, P. N., Walsh, W. J., Pogue, A. I., Percy, M. E., Kruck, T. P., Fang, Z., Scharfman, N., Jaber, V., Zhao Y., Li, W., Lukiw, W. J., (2018). Aluminum in neurologi cal disease–a 36-year multicenter study. Journal of Alzheimer’s disease & Parkinsonism, 8(6). https://doi.org/10.4172/2161-0460.1000457 DOI: https://doi.org/10.4172/2161-0460.1000457
Mold, M., Umar, D., King, A., & Exley, C. (2018). Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology, 46, 76-82. https://doi.org/10.1016/j.jtemb.201711.012 DOI: https://doi.org/10.1016/j.jtemb.2017.11.012
Liang, J., Liang, X., Cao, P., Wang, X., Gao, P., Ma, N., Li, N., & Xu, H. (2019). A preliminary investigation of naturally occurring aluminum in grains, vegetables, and fruits from some areas of China and dietary intake assessment. Journal of food science, 84(3), 701-710. https://doi.org/10.1007/s00217-018-3124-2 DOI: https://doi.org/10.1111/1750-3841.14459
Lopera, F. (2004). Enfermedad de Alzheimer. Perspectivas en Nutrición Humana, 29-32. https://doi.org/10.17533/udea.penh DOI: https://doi.org/10.17533/udea.penh
Luján, J. (2010). Ingesta de aluminio al cocinar alimentos y hervir agua con utensilios domés ticos. Tecnología y ciencia. Año 3, (6), 26-32. https://www.utn.edu.ar/images/Secretarias/SCTYP/revistas/Revista-a3n6.pdf
Martínez, D. B., Soldevilla, M. G., Santiago, A. P., y Martínez, J. T. (2019). Enfermedad de Alzheimer. Medicine-Programa de Formación Médica Continuada Acreditado, 12(74), 4338-4346. https://doi.org/10.1016/j.med.2019.03.012 DOI: https://doi.org/10.1016/j.med.2019.03.012
Matías-Cervantes, C. A., López-León, S., Matías-Pérez, D., y García-Montalvo, I. A. (2018). El aluminio empleado en el tratamiento de aguas residuales y su posible relación con enfer medad de Alzheimer. Journal of Negative and No Positive Results, 3(2), 139-143.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack Jr, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J.J., Mayeux, R., Mohs R. C., Morris, J. C., Rossor, M. N., Scheltens P., Carrillo, M.C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute Alzheimer’s disease and its association with dietary aluminum: a reviewGutiérrez-Álzate K, Acevedo-Correa D, Urzola-Ortega J, Fuentes-Berrio L, Beltrán-Cotta Lon Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’sdisease. Alzheimer’s & dementia, 7(3), 263-269. https://doi.org/10.1016/j.jalz.2011.03.005 DOI: https://doi.org/10.1016/j.jalz.2011.03.005
Mera, C. F., Gutiérrez, M. L., Montes-Rojas, C., y Paz J. P. (2016). Efecto de la moringa oleífe ra en el tratamiento de aguas residuales en el Cauca, Colombia. Biotecnología en el sector Agropecuario y Agroindustrial, 14(2), 100-109. https://doi.org/10.18684/BSAA(14)100-109 DOI: https://doi.org/10.18684/BSAA(14)100-109
Mirza, A., King, A., Troakes, C., y Exley, C. (2017). Aluminium in brain tissue in familial Alzheimer’s disease. Journal of Trace Elements in Medicine and Biology, 40, 30-36. https://doi.org/10.1016/j.jtemb.2016.12.001 DOI: https://doi.org/10.1016/j.jtemb.2016.12.001
Mol, S., & Ulusoy, S. (2020). The effect of cooking conditions on aluminum concentrations of seafood, cooked in aluminum foil. Journal of Aquatic Food Product Technology, 29(2), 186-193. https://doi.org/10.1080/10498850.2019.1707926 DOI: https://doi.org/10.1080/10498850.2019.1707926
Mukadam, N., Sommerlad, A., Huntley, J., & Livingston, G. (2019). Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health.;7:e596-603. https://doi.org/10.1016/S2214-109X(19)30074-9 DOI: https://doi.org/10.1016/S2214-109X(19)30074-9
Ning, M., Zhao-Ping, L., Da-Jin, Y., Jiang, L., Jiang-Hui, Z., Hai-Bin, X., Feng-Qin, L., & Ning, L. (2016) Risk assessment of dietary exposure to aluminium in the Chinese population, Food Additives & Contaminants: Part A, 33(10), 1557-1562. https://doi.org/10.1080/19440049.2016.1228125 DOI: https://doi.org/10.1080/19440049.2016.1228125
Oboh, G., Oladun, F. L., Ademosun, A. O., & Ogunsuyi, O. B. (2021). Anticholinesterase ac tivity and antioxidant properties of Heinsia crinita and Pterocarpus soyauxii in Dro sophila melanogaster model. Journal of Ayurveda and integrative medicine, 12(2), 254-260. https://doi.org/10.1016/j.jaim.2020.10.004 DOI: https://doi.org/10.1016/j.jaim.2020.10.004
Ogimoto, M., Suzuki, K., Haneishi, N., Kikuchi, Y., Takanashi, M., Tomioka, N., Uematsu, Y., & Monma, K. (2016). Aluminium content of foods originating from aluminium-containing food additives. Food Additives & Contaminants: Part B, 9(3), 185-190. https://doi.org/10.1080/19393210.2016.1158210 DOI: https://doi.org/10.1080/19393210.2016.1158210
Owodunni, A. A., & Ismail, S. (2021). Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. Journal of Water Process Engineering, 42, 102096. https://doi.org/10.1016/j.jwpe.2021.102096 DOI: https://doi.org/10.1016/j.jwpe.2021.102096
Peng, C. Y., Zhu, X. H., Hou, R. Y., Ge, G. F., Hua, R. M., Wan, X. C., & Cai, H. M. (2018). Aluminum and heavy metal accumulation in tea leaves: an interplay of environmental and plant factors and an assessment of exposure risks to consumers. Journal of food science, 83(4), 1165-1172. https://doi.org/10.1111/1750-3841.14093 DOI: https://doi.org/10.1111/1750-3841.14093
Rahman, M. A., Lee, S. H., Ji, H. C., Kabir, A. H., Jones, C. S., & Lee, K. W. (2018). Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: current status and opportunities. International journal of molecular sciences, 19(10), 3073. https://doi.org/10.3390/ijms19103073 DOI: https://doi.org/10.3390/ijms19103073
Ropers, M. H., Terrisse, H., Mercier-Bonin, M., & Humbert, B. (2017). Titanium dioxide as food additive. Application of Titanium Dioxide, 10. https://doi.org/10.5772/intechopen.68883 DOI: https://doi.org/10.5772/intechopen.68883
Sarkar, S., & Aparna, K. (2020). Food packaging and storage. Research Trends in Home Science and Extension AkiNik Pub, 3, 27-51.
Scheltens, P., Blennow, K., Breteler, M. M. B., de Strooper, B., Frisoni, G. B., Salloway, S., & Van der Flier, W. M. (2016). Alzheimer’s disease. The Lancet, 388(10043), 505–517. https://doi.org/10.1016/s0140-6736(15)01124-1 DOI: https://doi.org/10.1016/S0140-6736(15)01124-1
Silva, L. J., Pereira, A. R., Pereira, A. M., Pena, A., & Lino, C. M. (2021). Carmines (E120) in coloured yoghurts: a case-study contribution for human risk assessment. Food Additives & Contaminants: Part A, 38(8), 1316-1323. https://doi.org/10.1080/19440049.2021.1923820 DOI: https://doi.org/10.1080/19440049.2021.1923820
Silva, M. M., Reboredo, F. H., & Lidon, F. C. (2022). Food colour additives: A synoptical over view on their chemical properties, applications in food products, and health side effects. Foods, 11(3), 379. https://doi.org/10.3390/foods11030379 DOI: https://doi.org/10.3390/foods11030379
Stahl, T., Falk, S., Rohrbeck, A., Georgii, S., Herzog, C., Wiegand, A., Hotz, S., Boschek, B., Zorn, H., & Brunn, H. (2017). Migration of aluminum from food contact materials to food—a health risk for consumers? Part I of III: exposure to aluminum, release of aluminum, tole rable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environmental Sciences Europe, 29(1), 1-8. https://doi.org/10.1186/s12302-017-0117-x DOI: https://doi.org/10.1186/s12302-017-0116-y
Stahl, T., Falk, S., Taschan, H., Boschek, B., & Brunn, H. (2018). Evaluation of human exposure to aluminum from food and food contact materials. European food research and technology, 244(12), 2077-2084. https://doi.org/10.1007/s00217-018-3124-2 DOI: https://doi.org/10.1007/s00217-018-3124-2
Stephan, B. C. M., Hunter, S., Harris, D., Llewellyn, D. J., Siervo, M., Matthews, F. E., & Brayne, C. (2012). The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Molecular psychiatry, 17(11), 1056-1076. https://doi.org/10.1038/mp.2011.147 DOI: https://doi.org/10.1038/mp.2011.147
Takanashi, M., Ogimoto, M., Suzuki, K., Haneishi, N., Shiozawa, Y., Tomioka, N., Kimu ra, C., Okamura, R., Teramura, W., Uematsu, Y., Monma, K., & Kobayashi, C. (2018). Survey of Aluminium Content of Processed Food Using Baking Powder (2015-2016). Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 59(6), 275- 281. https://doi.org/10.3358/shokueishi.59.275 DOI: https://doi.org/10.3358/shokueishi.59.275
Teh, C. Y., Budiman, P. M., Shak, K. P. Y., & Wu, T. Y. (2016). Recent Advancement of Coagu lation–Flocculation and Its Application in Wastewater Treatment. Industrial & Engineering Chemistry Research, 55(16), 4363–4389. https://doi.org/10.1021/acs.iecr.5b04703 DOI: https://doi.org/10.1021/acs.iecr.5b04703
Toivonen, J., Hudd, R., Nystrand, M., & Österholm, P. (2020). Climatic effects on water quality in areas with acid sulfate soils with commensurable consequences on the reproduction of burbot (Lota lota L.). Environmental Geochemistry and Health, 42, 3141-3156. https://doi.org/10.1007/s10653-020-00550-1 DOI: https://doi.org/10.1007/s10653-020-00550-1
Trevizani, T. H., Domit, C., Vedolin, M. C., Angeli, J. L. F., & Figueira, R. C. L. (2019). Assessment of metal contamination in fish from estuaries of southern and southeas tern Brazil. Environmental monitoring and assessment, 191, 1-16. https://doi.org/10.1007/s10661-019-7477-1 DOI: https://doi.org/10.1007/s10661-019-7477-1
Troisi, J., Giugliano, L., Sarno, L., Landolfi, A., Richards, S., Symes, S., Colucci, A., Maruotti, G., Adair, D., Guida, M., Martinelli, P., & Guida, M. (2019). Serum metallome in preg nant women and the relationship with congenital malformations of the central nervous system: a case-control study. BMC Pregnancy and Childbirth, 19, 1-11. https://doi.org/10.1186/s12884-019-2636-5 DOI: https://doi.org/10.1186/s12884-019-2636-5
Villabona-Ortíz, A., Tejada-Tovar, C., & Contreras-Amaya, R. (2021). Electrocoagu-lation as an Alternative for the Removal of Chromium (VI) in Solution. Tecnura, 25(68), 28-42. https://doi.org/10.14483/22487638.17088 DOI: https://doi.org/10.14483/22487638.17088
Virk, S. A., y Eslick, G. D. (2015). Occupational exposure to aluminum and Alzheimer disease: a meta-analysis. Journal of occupational and environmental medicine, 57(8), 893-896. https://doi.org/10.1097/JOM.0000000000000487 DOI: https://doi.org/10.1097/JOM.0000000000000487
Wang, Z., Wei, X., Yang, J., Suo, J., Chen, J., Liu, X., & Zhao, X. (2016). Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neuroscience letters, 610, 200-206. https://doi.org/10.1016/j.neulet.2015.11.014 DOI: https://doi.org/10.1016/j.neulet.2015.11.014
Wang, Y., Lv, H., Lan, J., Zhang, X., Zhu, K., Yang, S., & Lv, S. (2022). Detection of Sodium For maldehyde Sulfoxylate, Aluminum, and Borate Compounds in Bread and Pasta Products Consumed by Residents in Jilin Province, China. Journal of Food Protection, 85(8), 1142-1147. https://doi.org/10.4315/JFP-22-011 DOI: https://doi.org/10.4315/JFP-22-011
Weidenhamer, J. D., Fitzpatrick, M. P., Biro, A. M., Kobunski, P. A., Hudson, M. R., Corbin, R.W., & Gottesfeld, P. (2017). Metal exposures from aluminum cookware: an unrecognized public health risk in developing countries. Science of the Total Environment, 579, 805-813. http://dx.doi.org/10.1016/j.scitotenv.2016.11.023 DOI: https://doi.org/10.1016/j.scitotenv.2016.11.023
Wen, Y., Huang, S., Zhang, Y., Zhang, H., Zhou, L., Li, D., ... & Cheng, J. (2019). Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environment international, 125, 125-134. https://doi.org/10.1016/j.envint.2018.12.037 DOI: https://doi.org/10.1016/j.envint.2018.12.037
WHO (2021). Informe sobre la situación mundial de la respuesta de la salud pública a la de mencia: resumen ejecutivo [Global status report on the public health response to demen tia:executive summary]. Ginebra: Organización Mundial de la Salud. https://apps.whoint/iris/bitstream/handle/10665/350993/9789240038707-spa.pdf [Cited on March 15thof 2023].
Yokel, R. A. (2016). Aluminum: Properties, Presence in Food and Beverages, Fate in Humans, and Determination. Encyclopedia of Food and Health, 128-134. http://dx.doi.org/10.1016/B978-0-12-384947-2.00023-4 DOI: https://doi.org/10.1016/B978-0-12-384947-2.00023-4
Zioła-Frankowska, A., D ˛abrowski, M., Kubaszewski, Ł., Rogala, P., y Frankowski, M. (2015). Factors affecting the aluminium content of human femoral head and neck. Journal of inor ganic biochemistry, 152, 167-173. https://doi.org/10.1016/j.jinorgbio.2015.08.01 DOI: https://doi.org/10.1016/j.jinorgbio.2015.08.019
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Recibido: 16 de septiembre de 2022; Aceptado: 24 de marzo de 2023
Abstract
Context:
Alzheimer is a neurodegenerative disease that not only occurs in the adult population. Some cases have also occurred in younger people. This has led to research relating to the ingestion of aluminum (considered a precursor to this disease) including its sources, which in many cases comes from food consumption.
Objective:
To conduct a literature review to provide an overview of Alzheimer’s disease and its relationship to dietary aluminum.
Methodology:
A literature review was carried out using the Scopus databases Science Direct, SpringerLink, Scielo, ResearchGate, Web of Science, and Google Scholar. In addition, information was obtained from websites.
Results:
Studies were found which associated aluminum intake in various forms with the onset of Alzheimer’s disease. Other studies demonstrated the presence of aluminum residue in various prepared foods through direct or indirect migration from utensils, water, or additives used in their preparation.
Conclusions:
It was identified that some foods can be a high source of aluminum intake due to leaching, direct absorption from the soil, or through the addition of this element via additives or colorants. This has raised awareness because of the link between this metal and Alzheimer’s disease.
Keywords:
contaminated food, dementia, health, heavy metal, migration, water.Resumen
Contexto:
El Alzheimer es una enfermedad neurodegenerativa que no solo se presenta en población adulta, sino que algunos casos también se han presentado en personas de menor edad. Esto ha llevado a que se realicen investigaciones relacionando la ingesta de aluminio (el cual es considerado un precursor de esta enfermedad) y su fuente de ingesta, que en muchos casos es provenientes del consumo de alimentos.
Objetivo:
Establecer mediante una revisión literaria una visión general de la enfermedad de Alzheimer y su relación con el aluminio consumido a través de la ingesta de alimentos.
Metodología:
Se realizó una revisión de literatura, usando como herramientas las bases de datos Scopus, Science Direct, SpringerLink, Scielo, ResearchGate, Web of Science e Google schoolar. Además, se contó con información proveniente de sitios web.
Resultados:
Se encontraron investigaciones donde se asocia la ingesta de aluminio en diferentes formas con la aparición de Alzheimer. Asimismo, se hallaron estudios en los cuales se demostraron la presencia de residuos de aluminio en distintos alimentos preparados, por la migración directa o indirecta de utensilios, agua o aditivos utilizados en su preparación.
Conclusiones:
Se pudo identificar que algunos alimentos pueden ser una alta fuente de ingesta de aluminio debida a la lixiviación, a la absorción directa del suelo o por la adición de este elemento a través de aditivos o colorantes. Esto ha generado conciencia debido a la relación existente entre este metal y la enfermedad de Alzheimer.
Palabras clave:
alimentos contaminados, demencia, salud, metal pesado, migración, agua.INTRODUCTION
Dementia is a disease which is a common and progressive neurological disorder primarily associated with older people (Kjaergaard et al., 2021). The prevalence and reporting of this disease varies according to the region of the world; countries with the best health care systems typically report a higher number of people suffering from this disease. According to data reported by the World Health Organization (WHO) in 2019, the highest registration of people with dementia occurred in the Pacific region (20.1 million reported cases), followed by Europe (14.1 million), the Americas (10.3 million), South-East Asia (6.5 million), the Eastern Mediterranean (2.3 million), and Africa (1.9 million) (WHO, 2021). The low registration of dementia cases in Africa may be associated with the poor healthcare systems in the majority of African countries. Currently, few countries give the necessary priority to dementia patients to access diagnostic and post-diagnostic services. This, coupled with low education, low socio-economic status, depression, social isolation, physical inactivity, smoking, hypertension, obesity, and diabetes, results in the under-recording of dementia cases (Mukadam et al., 2019).
Alzheimer’s is considered the most common neurodegenerative disease. Its main characteristics are gradual memory loss and impairment of cognitive functions, including attention span, language, and visuospatial skills (Ballard et al., 2016; Martinez et al., 2019). According to the American Alzheimer’s Association (2023), between 2000 and 2017, the number of reported deaths from Alzheimer’s disease (AD) compared to the number of deaths from stroke, heart disease, and prostate cancer increased by 145 %. In 2019, AD was the sixth leading cause of death in the United States and the fifth leading cause of death among Americans aged 65 and older, according to official death certificates. However, between 2020 and 2021, it was the seventh leading cause of death due to deaths from SARS-CoV-2. Currently, approximately 6.7 million people have AD; this number may increase to 7.2 million in the United States by 2025, while it is expected that there will be 115 million people with AD worldwide by the year 2050. AD is not a disease that only affects older individuals, as there have been cases where this disease begins in individuals with an average age of 50 years. In these cases, it is unknown whether there was a genetic predisposition. Studies which included cases of this type have found that the affected subjects have been exposed occupationally (Exley et al., 2014; Mirza et al., 2016) or environmentally (Exley and Esiri, 2006) to high levels of aluminum for prolonged periods. These results have allowed researchers to identify the link between exposure to this element with the onset of AD (Exley, 2017).
Aluminum is one of the most abundant chemical elements in nature, being a metal with proinflammatory, pathological, and genotoxic characteristics which is especially harmful to the homeostatic performance of brain cells, particularly as concerns normal genetic and cytoplasmic functioning. This metal migrates to humans through the consumption of water, contaminated food, and/or processed foods that contain alumimum which is frequently used in packaging and preservatives and/or colorants, the latter being the most bioavailable form absorbed by the intestine. The consumption of food contaminated with aluminum can gradually lead to memory loss. It should be noted that the maximum tolerable level of aluminum exposure is 1 mg aluminum/kg body weight/week (Crisponi et al., 2013; Bondy, 2016; Matías-Cervantes et al., 2018).
Considering the relationship between aluminum and AD, studies have been developed such as those conducted by Matias-Cervantes et al. (2018), Mera et al. (2016), Arcila and Peralta (2016) and Ekong et al. (2017), evidencing that treated water could be contaminated with aluminum, this being one of the main routes of intake. This has led to new research on the use of vegetable coagulants obtained from seeds (moringa, jatropha curcas, nirmali), peels, rinds, and skins (banana, watermelon, beans, cassava, papaya, okra), fruit waste, and other natural sources to reduce the use of traditional aluminum-based coagulants (alum, sodium aluminate, aluminum chloride, polyaluminum chloride, polyaluminum chlorosulfate, and polyaluminum sulfate) due to their associated health hazards (Teh et al., 2016; Owodunni & Ismail, 2021). Therefore, the purpose of this research was to conduct a literature review to provide an overview of Alzheimer’s disease and its relationship to dietary aluminum.
METHODOLOGY
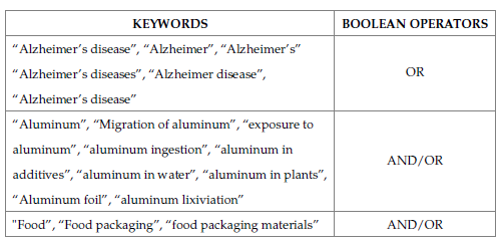
This article is based on a literature review exploring the relationship between aluminum from food or the food industry and AD, which was conducted by searching for publications available in the databases Scopus, Science Direct, SpringerLink, Scielo, ResearchGate, Web of Science, and Google scholar. In addition, information was obtained from websites. The books, articles, and other research sources consulted for this review were all published between 2003 and 2023. For the above, keywords and boolean operators were used (Table 1).
Sources: Authors
Table 1: Terms and Boolean operators used for literary review
Background
Research on AD began when Dr. Alois Alzheimer discovered histological alterations in the brain of a 51-year-old woman in the late 1880s. In 1906, Dr. Alzheimer, during a lecture in Tubingen (Germany), presented his first observations on the symptoms and pathology of this disease (Hippius and Neundörfer, 2003; Lopera, 2004). In 1907, Dr. Alzheimer formally established the existence of a new disease, which was initially considered a strange form of presenile dementia characterized by behavioral disorders, depression, psychotic symptoms, and cognitive impairment. At the time, these symptoms were considered strange because the main origin of dementia was believed to be the "hardening of the arteries". In 1910, Emil Kraepelin assigned the name .Alzheimer’s disease"to this pathology, a disease which was a significant burden for patients, their caregivers, and the community at large (Hippius & Neundörfer, 2003). Subsequent advances in neuropathology allowed AD to be considered a type of presenile dementia distinct from senile dementia. This hypothesis prevailed until the 1960s, and for the next 20 years, AD remained the dominant and prototypical form of dementia, centered on prominent memory disturbances. However, the term dementia has gradually fallen out of use when referring to major neurocognitive disorder and the heterogeneity of the syndrome at the phenotypic and molecular levels has once again been recognized (Assal, 2019).
Diagnosis of Alzheimer’s disease
A definitive diagnosis of AD should include multiple types of progressive cognitive impairments leading to dementia with post-mortem neuropathologic confirmation, as well as a clinical history of memory impairment. Recently, the importance of the use of biomarkers that aid in the in vivo diagnosis of AD has been accepted. Among these markers, one can find the main cerebrospinal fluid (CSF) biomarkers of AD, namely are amyloid-beta (Aβ42), which shows the cortical deposition of amyloid, total tau (t-tau), which reflects the intensity of neurodegeneration, and phosphorylated tau (p-tau), which correlates with pathological neurofibrillary changes. Magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG) positrón emission tomography (PET) techniques have also been implemented to discard intracranial causes (meningioma, subdural hematoma) and have been complemented by the notion that the demonstration of regional atrophy in the medial temporal region can provide positive diagnostic information (Scheltens et al., 2016).
MRI remains the modality of choice for the assessment of cerebral vascular changes, such as white matter hyperintensities, lacunae, and microbleeds, which have gained increasing attention because these are frequent secondary effects in antiamyloid trials. Meanwhile, FDG PET estimates the density and distribution of aggregated tau neurofibrillary tangles in cognitively impaired adults being evaluated for AD. Precise interpretation of FDG with PET in patients with dementia is not based on the presence or absence of a single region of hypometabolism, but should take into account the pattern of hypometabolism throughout the cortex (Scheltens et al., 2016; Barthel, 2020). However, further standardization is required to have universal clinical use of biomarkers (McKhann et al., 2011; Barthel, 2020). Likewise, although age is one of the primary factors associated with AD, there is a wealth of research which currently points to neurodegeneration, inflammation, atrophy, and other elements of concern as chronic circumstances that may favor the manifestation of dementia as well as the development of lesions in the structures of the medial temporal lobe, hippocampus, and entorhinal cortex. These lesions are characteristic of the anatomopathological alterations typical of AD due to Aβ protein plaques outside neurons and Tau protein neurofibrillary tangles inside neurons, physiological characteristics of a patient which usher the progression of the disease (Stephan et al., 2012).
General information on aluminum
Aluminum is the third most abundant chemical element in the earth’s crust, making up 7.5%of its composition. Despite its prevalence, it has no biological function in human or animal organisms. However, its low density and ability to self-passivate cause it to be widely used in industries (Stephan et al., 2012; Stahl et al., 2017).
According to the European Aluminum Foil Association, aluminum has been used commercially for more than a century.
Due to its abundant availability and characteristics, since the late 19th century, it has increasingly shaped the modern lifestyle and has become necessary for space exploration, electricity transmission, the construction of modern buildings, and the manufacturing of aircrafts, automobiles, vessels, and currently, high-quality packaging of various types for the food industry, to which it is especially suited given its low cost and high levels of conductivity which enable effective temperature regulation (Casaburi et al., 2019).
Therefore, the production of primary aluminum has increased significantly in recent years, reaching a worldwide production level of 68,461 thousand metric tons in 2022, with China being the largest producer at 40,430 thousand metric tons (International-Aluminum, 2023). However, alloying additives and recycled aluminum, which are excluded from the processing of primary aluminum, are used in the production of secondary aluminum, a process which can be repeated almost indefinitely, which cuts costs and multiplies environmental benefits. Aluminum figures are thus expected to increase considerably due to advances in aluminum alloy metallurgy (Brough & Jouhara, 2020).
Sources and behavior of aluminum in food
The migration of aluminum into food impacts the health of consumers, bringing as consequences neurological diseases involving inflammatory neural degeneration, behavioral deterioration, and cognitive impairment (Barthel, 2020). In addition, its accumulation in the central nervous system (CNS) over time can lead to irreversible brain cell damage and functional decline resulting in cognitive, memory, and behavioral deficits. Consequently, researchers have analyzed the aluminum content of the temporal lobe neocortex, finding a range of 1.9 16.8 ug aluminum/gm tissue in autopsies performed on patients whose cause of death was AD (McLachlan et al., 2018).
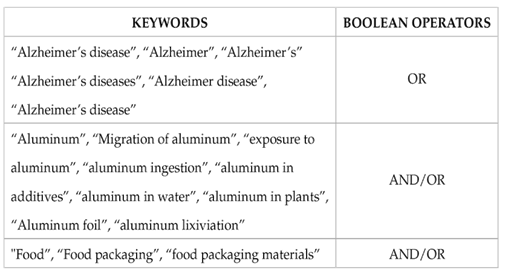
One of the causes of aluminum accumulation in the human body is the consumption of foods which contain this element, either from primary or secondary sources. Primary aluminum in food is generated by the natural migration of aluminum from the earth’s crust (where this element is present) to food. This phenomenon occurs primarily in plants. Plants absorb nutrients from the soil that are used for nutrition, development, and growth. Aluminum can be found in fresh vegetables, with values between 2 and 10 mg/kg, depending on the type of soil (alkaline or acidic) where it is harvested, the concentration of aluminum present in the soil, the water used for irrigation, and the type of vegetable, leafy vegetables having the highest values, followed by bulb, stem, flower, pod, root, and tuber vegetables. In fruits, on the other hand, concentrations are lower (mean value of aluminum 1.3 mg/kg) (Liang et al., 2019; Daouk et al., 2020).
Estimates suggest that 40-50% of cultivable soils worldwide are acidic (pH <5.0). Here, aluminum is present in a cationic form (being highly soluble) which allows plants to easily obtain a higher concentration (Rahman et al., 2018). However, cereal plants are among the plants with the lowest aluminum accumulation. Levels of aluminum in cereal plants are also highly variable between countries or even regions of the same country, as shown in Table 2, which outlines the presence of this metal not only in grains but also in other parts of plants (leaves and shoots) and cereal-based products.
Table 2: Aluminum concentration (mg/kg) in some grains, parts of plants, or cereal products
Soil geochemistry not only affects plants and plant products through aluminum migration but also alters water quality. This contamination causes water quality to decrease to levels prejudicial to biota in waterways affected by the easy discharge of aluminum due to the high solubility of this contaminant in more acidic conditions such as those in acidic soil and rain (acid rain) (Hu et al., 2017; Toivonen et al., 2020). Therefore, the above-mentioned phenomena may be factors in the increased levels of aluminum in some plants, as is the case of tea plants (Camellia sinensis) which are produced in acid soils with a pH range of 3.5 5.6 (De Silva et al., 2016; Hu et al., 2017). Research has reported aluminum concentrations in tea leaves between 1836.77 and 487.57 mg/kg (Peng et al., 2018; de Silva et al., 2016; Li et al., 2015), which has generated research to address the management and control of aluminum levels in soils and crops. Reducing these levels could help counteract the concentrations present in water systems. Besides coming from areas with acidic rocks and soils, this metal can also be found in nature, in lake water, either as untreated water or as water treated with Al salts or with electrocoagulation/ electroflotation. The latter is a recognized decontamination method for water treatment in which aluminum electrodes can be used wherever the release of aluminum metal molecules may occur to decontaminate the water containing dyes (Garcia et al., 2016). Moreover, these electrodes are used to remove heavy metals such as chromium present in water (VillabonaOrtíz et al., 2021). Salts are also inappropriately used as coagulants in drinking water treatment to reduce organic matter, color, turbidity, and microorganism levels. In this way, aluminum is transported via the liquid until it reaches residences where it is sometimes consumed directly from the tap. This water could contain a higher aluminum content after purification with aluminum salts since this compound increases the percentage of dissolved polyaluminum species (Al Zubaidy et al., 2011; D’Haese et al., 2019).
This can cause a risk to human health, especially when aluminum is present in high concentrations (≥ 0.1 mg/L aluminum) as it becomes a catalyst for AD and dementia (FAO/WHO, 2007; Matías-Cervantes et al., 2018). Similarly, water treatment affects the aquatic ecosystem by generating contamination with non-essential oligoelements, mainly aluminum, which is transferred through the trophic chain to crustaceans and especially fish. The latter has been held to be amongst the most susceptible aquatic organisms to the accumulation of metals. Consequently, these marine species become vectors of metal contamination for humans which can cause health risks when levels of toxic elements are very high (Trevizani et al., 2019; Dos Santos et al., 2023). Therefore, research has focused on finding natural alternatives to reduce the use of aluminum salts as flocculants in drinking water purification (Ekong et al., 2017; Chao et al., 2020).
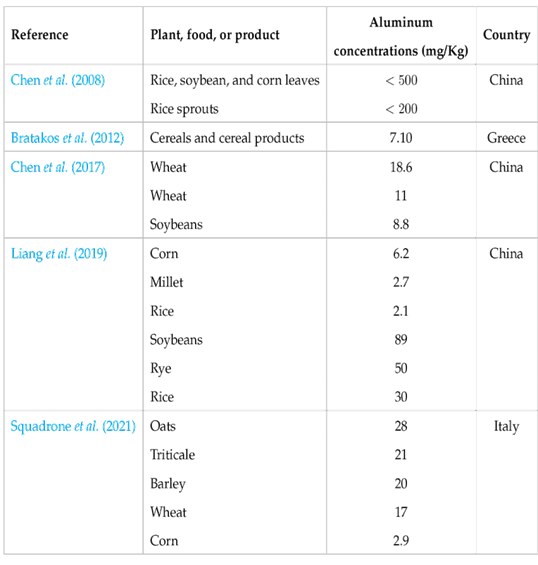
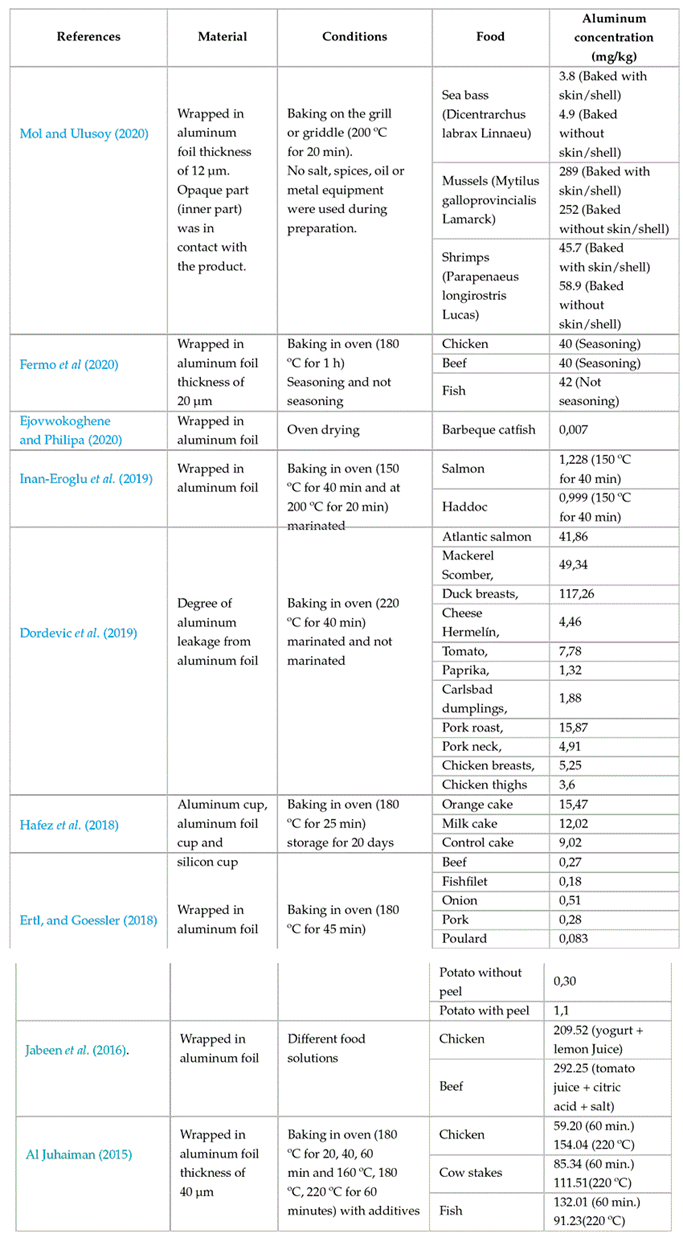
Another way in which humans can be exposed to aluminum is through the ingestion of aluminum through food contaminated during processing, packaging, and/or storage. This is secondary source of aluminum associated with the lixiviation of this element from kitchen equipment and utensils (pots, cutlery, trays, knives, frying pans, grills, etc.) that are manufactured with the material. Aluminum is widely used in the industry for the production of these utensils mainly because it is an easily obtainable material with good malleability, thermal conductivity, ease of clean up, and durability (Odularu et al., 2013; Stahl et al., 2018). Despite these industrial benefits, aluminum can easily leach into food due to factors such as the type of aluminum utensils used, exposure time, cooking temperature, salinity, pH, fat content, and food composition in general (Al Zubaidy et al., 2011; Bassioni et al., 2012). Another secondary factor of aluminum exposure is the use of aluminum foil in food preparation and culinary practices. Aluminum foil has been widely used to wrap heat-sensitive foods (mainly seafood and meat products) before cooking, which can generate a high concentration of aluminum in the producto after heating.
Table 3 shows studies that have evaluated the leaching of aluminum from aluminum foil into various foods. It shows that the amount of leaching can increase depending on the characteristics of the food, pH, whether it is marinated (wines, citric acid, tomato juice, apple cider vinegar), acidity, or whether it contains spices or additives. Here, acidic solutions and spices, along with increased temperature and cooking time, contributed to increased leaching of the aluminum exposure area. Weidenhamer et al. (2016) studied the release of aluminum from cookware materials in 10 developing countries (Bangladesh, Guatemala, India, Indonesia, Ivory Coast, Kenya, Nepal, Philippines, Tanzania, and Vietnam), finding that there was an average aluminum exposure for all cookware studied of 125 mg per serving, six times higher than the provisional tolerable weekly intake for a 70-kg adult (20 mg/day). These authors made preliminary evaluations of three possible methods to reduce metal leaching in the cookware tested (boiling or near-boiling water, addition of curcumin, and fluoropolymer coating), where the fluoropolymer coating reduced up to 98% of the aluminum level in the final extraction.
Table 3: Leaching of aluminum from food through food preparation and storage.
Likewise, trays or containers can be found in the market, which are made of aluminum sheets with thicknesses greater than those used to make paper; these are mainly designed for solid and/or semi-solid products. Therefore, this material has attracted much interest in the industry as it is an excellent barrier against gases, light, moisture, odors, flavors, and microorganisms and has properties of impermeability, resistance to freezing, inertness, especially perfect dead fold characteristics, and recyclability (Sarkar & Aparna, 2020). However, it can pose risks to human health due to the migration that occurs when the material comes into contact with food and is exposed to heat. This can lead to corrosion and erosion of the container, allowing aluminum to leach into the food and then follow the digestive and circulatory tract, and finally, it is stored in the tissues, including the brain; similar process happens with aluminum containers, traces of this metal are extracted from the walls of the container, it passes to the liquid phase and followed by the product, presenting itself in low pH sauces and fruit juices (Bejarano & Suárez, 2015; Deng et al., 2021).
Aluminum can also be found as a secondary source of food additives. These are substances that are intentionally added to food for a technological purpose in the manufacture, preparation, processing, treatment, packaging, wrapping, transport, or preservation of the food, so the use of Al-containing additives can affect the total concentration in the final product (Yokel, 2016; FAO/WHO, 2021). In the additives market, aluminum can be found as an ingredient in several of these compounds, such as sodium aluminum sulfate (sodium aluminum dúplex sulfate, E521, INS 521), potassium aluminum sulfate (potassium alum, E522, INS 522), ammonium aluminum sulfate (ammonium alum, E523, INS 523), potassium aluminum sulfate (potassium alum, E522, INS 522), ammonium aluminum sulfate (ammonium alum, E523, INS 523), sodium aluminum phosphate (aluminum salt and phosphoric acid salt, E541i, INS 541i), sodium aluminosilicate (sodium aluminum silicate, E554, INS 554), calcium aluminum silicate (E556, INS 556), and aluminum silicate (E559, INS 559). These additives are technically used in processed products and water as synthetic stabilizers, coagulants, and leavening agents to control the rate of CO2 gas generation, emulsifiers, and acidity regulators (Ogimoto et al., 2016; FAO/WHO, 2021). Among the above-mentioned additives are included fermenting agents, demolding agents, anti-caking agents, and protectants such as aluminum and sodium silicate in cake mixes and dry products, gelatins, wheat flour, and wheat-based foods (including fried dough sticks, fried dough cakes, steamed bread, noodles, cakes, and pastries, etc.). These are the foods with the highest levels of aluminum and potassium dodecahydrate due to the use of aluminum and potassium sulfate dodecahydrate, fried dough cakes, steamed bread, noodles, cakes, and pastries, etc.) are the foods with the highest levels of aluminum due to the use of aluminum potassium sulfate dodecahydrate (alum) in the preparation of these types of products (Ning et al., 2016; Wang et al., 2022). Due to the health risks posed by aluminum, some countries, such as China, have conducted several studies (Guo et al., 2015; Chen et al., 2017; Ding et al., 2021) on the use of additives containing this metal to provide scientific information to the government so that it can better control the limits of aluminum residues in food additives.
In the food industry, aluminum is also used as a colorant (E173), known as Çl pigment metal", which presents a silver-gray hue or tiny sheets used to decorate bakery and confectionery products (Silva et al., 2022). It is also used in the extraction process of some colorants such as carmine (carminic acid E120), which is obtained naturally from cochineal when it is subjected to a heat treatment and pH 5 in combination with aluminum, citric acid, and calcium salts (Gebhardt et al., 2020). This colorant is commonly used in the production of meat products (smoked fish, crustacean paste, fish paste, pre-cooked crustaceans), dairy products (yogurts, ice creams, fresh flavored cheeses, cured cheeses, edible cheese rinds), among others (candies, sweets, candies, chewing gum, desserts, cakes, pastries, candies, jams, vitamins, pharmaceutical tablets, and medicinal capsules), due to the tonality (purple to red) that it imparts in the product mainly used (Silva et al., 2021). Another colorant extensively used to improve the appearance of soft drinks, dairy products, candies, and confectionery, are anthocyanins (E163) which can be obtained from vegetables and edible fruits, such as blueberries, strawberries, raspberries, blackberries, currants, and grapes. In this colorant can be found aluminum particles product of the extraction of this compound (Gebhardt et al., 2020; Silva et al., 2022). Also, aluminum in the form of aluminum oxide is used to improve the technological properties of titanium dioxide or ”CI white pigment 6", a colorant that is used in confectionery products, decorations, dairy products and analogs, surimi and similar products, salmon substitutes, seasonings, condiments, mustard, sauces, broths, soups, among others (Ropers et al., 2017; Silva et al., 2022).
In general, the use of aluminum-containing additives is gaining increasing significance due to the technological benefits they offer in terms of their visual impact on food. Sometimes these additives enhance the food’s color, making it much more attractive than its natural hue. Additionally, some additives like sodium aluminum silicate, calcium aluminum silicate, and aluminum silicate, serve as anti-caking agents in dry powdered products and generate greater solubility at the time of preparation (FAO/WHO, 2021). Sodium aluminum phosphate is also used to emulsify and improve product quality, melt cheese, thicken juices and sauces, and for pickling vegetables, and is found in fruit confectionery, meat binders, dough reinforcers, stabilizers, buffers, neutralizers, texturizers, and curing agents (Ogimoto et al., 2016). Likewise, the additives aluminum sodium sulfate, aluminum potassium sulfate, and aluminum ammonium sulfate are used to regulate acidity in order to reduce the growth of organisms both in water and in foods, mainly of vegetable origin, resulting in them being used as preservatives. Therefore, these additives function in ways that both appeal to consumers and, at the same time, lead to better results for the industry due to the profitability of these foods on the market. Thus, in some countries, attempts to ban the use of additives which contain aluminum have not been successful, which has led to the regulation of the use of each additive based on the concentration of aluminum it contains. This includes stipulations that products must be labeled with the type of additive used according to the specifications established by the Codex alimentarium
(Ropers et al., 2017; FAO/WHO, 2021).
Adverse effects of aluminum on human health
According to WHO, the tolerable daily intake (TDI) of aluminum for humans should be 1 mg/kg body weight/day (FAO/WHO, 2007). Exposure at these levels is not a problem since the human body can excrete small amounts of this metal very efficiently. Unfortunately, a large part of the population is exposed to and ingests more than their bodies can excrete. As a result, the effects that this metal can produce on tissue function can be significant, starting with the reduction of human brain cell growth, proportional to the amount of concentrated aluminum (Diamond, 2008; Bassioni et al., 2012). The above has spurred a surge in research on the relationship between the accumulation of aluminum in the human body and the risk of multiple neurological pathologies. Some studies, cited in Table 4, were carried out to determine the levels of aluminum present in patients diagnosed with neurological pathologies such as autism spectrum disorder, the precise origins of which are unknown. Mold et al. (2018) performed the first study which examined the aluminum content in the brain tissue of people diagnosed with autism. The results showed a high presence of aluminum in the extracellular and intracellular tissue, suggesting that aluminum may be related to the etiology of this disorder. Likewise, aluminum has been associated with breast cancer due to the accumulation of traces from the use of cosmetic products such as deodorants that may contain aluminum salts. In the cases studied, women and the elderly were found to be the most susceptible to the accumulation of metals in their bodies as a result of the use of some medications, environmental exposure, water intake, and foods with high concentrations of additives such as those mentioned in this study. Therefore, it is important to give consumers the information necessary to make informed decisions about the type of products consumed and the type of utensils used for their preparation. This is especially important for pregnant women, since this metal may from an early age, and research has even established that aluminum may be related to congenital malformations of the central nervous system (Troisi et al., 2019).
Table 4: Research on the relationship between aluminum and human health risks
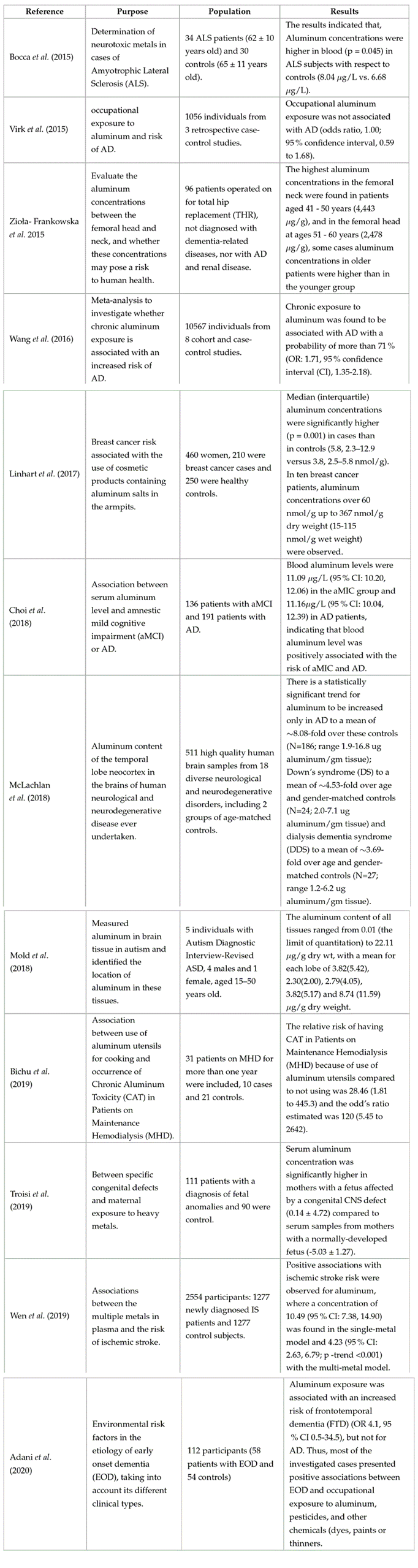
Also in the studies mentioned in Table 4, AD is one of the most studied pathologies because of the relationship aluminum has with the neurotoxin associated with the disorder. The research indicates that the abnormal aluminum concentrations found in elderly patients, as well as in young patients with AD, can cause an excess of inflammatory activity in the brain, which is a factor that accelerates the rate of brain aging, in turn inevitably increasing the incidence of age-related neurological diseases (Bondy, 2016).
According to Exley (2017), AD is considered an acute response to chronic aluminum intoxication, with aluminum acting as a catalyst in the early onset of the disease because of the way the brain responds to this aluminum load. This metal is accumulated in the body in the frontal cortex and hippocampal regions of the brain, generating neurotoxic activities in the central nervous system which lead to decreased enzymatic activities, increased oxidative stress, and aggregation of proteins such as beta-amyloid (Aβ), all of which contribute to the generation of senile plaques where a series of processes can lead to neurodegeneration and cell death (Kabir et al., 2020).
Research on aluminum from cookware and aluminum foil
One of the sources of aluminum contamination in food is cooking equipment such as pots and pans, among others, which, when subjected to high temperatures (>100 ºC), undergo a leaching process, causing the food to absorb traces of aluminum which are subsequently ingested by the consumer. In 2010, Luján studied the aluminum ingestion that occurs when boiling water and cooking food in pots made with aluminum. The results found that aluminum was present in the water and food (vegetable soup) after 30 minutes of heating at concentrations of 220 µg/L for the water and 400 µg/L for the soup. In another study, Cisneros et al. (2019) found a range between 2.33 and 5.12 mg/kg of aluminum in rice cooked in 6 containers from different brands, which exceeds the limits set out in European regulations of 1 mg/kg.
Another source of contamination by this element is aluminum foil, which is widely used to wrap food for cooking or reheating, either in the oven or using pots or pans. To address this concern, Ertl and Goessler (2018) evaluated the aluminum content in foods that were coated with aluminum foil and baked at 5 min at 180 °C. Notably, the results revealed a 12-fold increase of aluminum in cow (from 0.021 to 0.27 mg/kg), a 5-fold increase in onion (from 0.088 to 0.51 mg/kg), and a 4-fold increase in pork (from 0.055 to 0.28 mg/kg), after the baking process. These findings provide clear evidence of the metal leaching into the food after being subjected to a thermal process. In this same research, it was found that high temperatures are not the only cause of aluminum leaching into foods. After 3 days of refrigeration at 7 ºC, foods such as salmon, ham, lemon, and orange increased their aluminum content 16 times (from 0.13 to 2.2 mg/kg), 32 times (from 0.11 to 3.6 mg/kg), 163 times (from 0.032 to 5.2 mg/kg), and 200 times (from 0.034 to 6.9 mg/kg) respectively.
Likewise, Ejovwokoghenea and Philipa (2020) investigated the risk of aluminum consumption through the ingestion of barbecued catfish that was wrapped in aluminum foil for protection during cooking. These researchers found that the fish went from 0.039 mg/kg (raw) to 0.047 mg/kg after the cooking process, which indicated the rate of leaching of 18.65 % aluminum into the fish. However, consuming this food in small quantities, prepared as described here, does not pose a danger, as the aluminum content remains below the amount allowed by the norms (1 mg/kg).
On the other hand, the presence of aluminum can also be caused by migration from the food packaging. Iscuissati et al. (2021) evaluated the migration of aluminum to coffee prepared by a high-pressure machine with metal seals (Nespresso® Essenza Mini machine). It was found that using the machines to prepare the beverages contributes to increase the aluminum content by approximately 13 % (459 µg/L) compared to a conventional filtration coffee preparation process (408 µg/L). Concerns are also expressed about the reuse of ground coffee, since the increase in aluminum content is approximately 3.5 times higher in this after its preparation, and therefore this recycling strategy should be discarded.
For this reason, Stahl et al. (2018) investigated human exposure to aluminum and food contact materials. The study found regional differences that led to variations in the global consumption of aluminum. For the adult population, the average exposure to this metal was between 0.2 and 1.5 mg/kg body weight/week. On the other hand, children and adolescents, who have a lower body weight, were found to have a higher aluminum concentration (between 0.7 and 2.3 mg/kg body weight/week). These results show values between 14 and 105 mg of aluminum/week for an adult weighing 70 kg while the values for a child weighing 30 kg were 21 to 69 mg aluminum/week. These values indicate that a portion of the human population can consume enough aluminum through their usual diet to reach the tolerable weekly intake.
Between 2015 and 2016, Takanashi et al. (2018) conducted a survey in Japan on the aluminum content in flour-based products and confectionery with baking powder. Aluminum was found in 33.33 % of the evaluated products (corresponding to 41 out of 123 samples), at levels between 0.01 (limit of quantification) and 0.40 mg/g. The presence of this metal in confectionery products was reduced compared to previous studies. However, the presence of aluminum was high in Japanese confectionery and flour-based foods. Consuming one serving of 4 of the 41 samples analyzed would result in an aluminum intake that exceeds the recommended levels for young children, whose average weight was 16 kg.
To study the toxic risk of aluminum intake, Hardisson et al. (2017) collected and compared data on the concentrations of this metal across various types of foods, aiming to estimate the total dietary intake. The most predominant analytical techniques for aluminum determination were inductively coupled plasma atomic mass spectrometry and atomic emission spectroscopy (ICP-OES and ICP-AES). The highest aluminum levels were found in vegetables (16.8 mg/kg), fish and shellfish (11.9 mg/kg), and roots and tubers (9.60 mg/kg). Among the foods that contributed most to the tolerable weekly intake of this metal were fruits (18.2 % for adults and 29.4 % for children) and vegetables (32.5 % for adults and children). As a result, it could be concluded that the dietary intake of aluminum may pose a health risk due to the accumulation of this metal in the brain caused by long-term intake.
Plant extracts to fight Alzheimer’s disease
Considering the need of reducing the effects generated by aluminum on the nervous system, researchers have been driven to explore the potential of the Moringa oleifera plant. Ekong et al. (2017) studied the neuroprotective effects of moringa leaf extract on aluminum-induced temporal cortical degeneration in rats and concluded that it protects against aluminum-induced neurotoxicity of the temporal cortex of rats.
Previous research has confirmed that yerba mate (Ilex paraguariensis) has an antioxidant potential that could help reduce the risk of developing neurodegenerative diseases, such as AD; antioxidants can mitigate the oxidative stress that causes and/or contributes to the development or progression of AD. Bortoli et al. (2018) evaluated the potential of I. paraguariensis in the etiology of AD using Caenorhabditis elegans strains. The study explored the concentration of aluminum and antioxidants in the plant’s leaf extract. It was determined that the metal content impacts the Acetylcholinesterase (AChE) activity. Consequently, acute and chronic exposure to both the element and leaf extract of I. paraguariensis demonstrated notable similarity to wildtype worms. In addition, it was observed that the results in both transgenic strains exposed long-term to leaf extract and aluminum concentrations showed an increase in AChE activity.
Similarly, Elufioye et al. (2019) found that extracts from the leaves of Macrosphyra longistyla have high antioxidant and anticholinesterase activities. According to the results found, extracts from this plant can potentially be used in the treatment of neurodegenerative diseases such as AD. Similarly, Oboh et al. (2021) found that extracts from the leaves of Heinsia crinita and Pterocarpus soyauxii, owing to their anticholinesterase, antioxidant and metal chelating properties, can reduce the presence of aluminum in the body. Researches have shown that the use of extracts from natural sources (mainly plants like the aforementioned) can be an alternative against AD, due to the multiple benefits they offer.
CONCLUSIONS
The results of the research in this area provide important information to consider that AD is a degenerative disease and may be related to the accumulation of aluminum in the brain. This metal is accumulated in the human body mainly through the ingestion of foods contaminated with aluminum; being a heavy metal, it is often found in the soil and is therefore easily present in fruits and vegetables, as well as in water. In addition, the use of containers or kitchen utensils made of aluminum becomes a source of contamination of products since the migration process of this metal is accelerated when exposed to heat in contact with food. In the last few years, the population has become increasingly aware of the health risks caused by the ingestion and accumulation of this metal in the human organism. The concern generated by the consumption of aluminum through food matrices and its consequences on the health of consumers has led researchers, industry, and consumers to develop and use alternatives for kitchen utensils, packaging, food additives, and water treatment with materials that do not contain this metal, with the purpose of mitigating the risks of aluminum consumption and its relationship with AD.
REFERENCES
License
Copyright (c) 2023 Katherine Gutiérrez-Álzate, Diofanor Acevedo-Correa, Jefferson Jose Urzola-Ortega, Lorenzo Fuentes-Berrio, Luis Alfonso Beltrán-Cotta

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Esta licencia permite a otros remezclar, adaptar y desarrollar su trabajo incluso con fines comerciales, siempre que le den crédito y concedan licencias para sus nuevas creaciones bajo los mismos términos. Esta licencia a menudo se compara con las licencias de software libre y de código abierto “copyleft”. Todos los trabajos nuevos basados en el tuyo tendrán la misma licencia, por lo que cualquier derivado también permitirá el uso comercial. Esta es la licencia utilizada por Wikipedia y se recomienda para materiales que se beneficiarían al incorporar contenido de Wikipedia y proyectos con licencias similares.

