DOI:
https://doi.org/10.14483/udistrital.jour.colomb.for.2015.1.a09Published:
2015-01-01Issue:
Vol. 18 No. 1 (2015): January-JuneSection:
Scientific articleEpizoochory in dry forest iguanas: an overlooked seed dispersal mechanism?
Epizoocoría por medio de iguanas en el bosque seco: ¿un mecanismo de dispersión de semillas pasado por alto?
Keywords:
epizoochory, Melocactus curvispinus, saurochory, Tatacoa, tropical dry forest (en).Keywords:
epizoochory, Melocactus curvispinus, saurochory, Tatacoa, tropical dry forest. (es).Downloads
References
Andersen, E. (1999). Seed dispersal by monkeys and the fate of dispersed seeds in a Peruvian rain forest. Biotropica, 31,145-158.
Bazzaz, F. A., Levin, D. A. & Schmierbach, M. R. (1982). Differential survival of genetic variants in crowded populations of Phlox. Journal of Applied Ecology, 19, 891-900.
Benítez-Malvido, J., Tapia, E. Suazo, I., Villaseñor, E. & Alvarado, J. (2003). Germination and Seed Damage in Tropical Dry Forest Plants Ingested by Iguanas. Journal of Herpetology, 37 (2), 301-308.
Bullock, J.M., Galsworthy, S.J., Manzano, P., Poschlod, P., Eichberg, C., Walker, K. & Wichmann, M.C. (2011) Process-based functions for seed retention on animals: a test of improved descriptions of dispersal using multiple data sets. Oikos, 120, 1201–1208.
Burgin, S. & Renshaw, A. (2008). Epizoochory, Algae and the Australian Eastern Long-Necked Turtle Chelodina Longicollis (Shaw). The American Midland Naturalist, 160(1), 61-68.
Figueroa, Y.C. & Galeano, G. (2007). Lista comentada de las plantas vasculares del enclave seco interandino de la Tatacoa (Huila, Colombia). Caldasia, 29 (2), 263-281.
Castilla, A.M. (1999). Podarcis lilfordi from the Balearic islands as a potential disperser of the rare Mediterranean plant Withania frutescens. Acta Oecologica,
(2), 103-107.
Cavelier, J., RUIZ, A., Santos, M. Quiñones, M. & Soriano, P. (1996). El proceso de degradación y sabanización del Valle Alto del Magdalena. Informe inédito, Fundación del Alto Magdalena. Bogotá.
Celedón-Neghme, C. L. San Martin, A., Victoriano, P. F., & Cavieres, L. A. (2008). Legitimate seed dispersal by lizards in an alpine habitat: The case of Berberis empetrifolia (Berberidaceae) dispersed by Liolaemus belii (Tropiduridae). Acta oecologica, 33, 265–271.
Couvreur, M., Verheyen, K. & Hermy, M. (2005) Experimental assessment of plant seed retention times in fur of cattle and horse. Flora, 200, 136–147.
Fialho, R.F. (1990). Seed dispersal by a lizard and a treefrog effect of dispersal site
on seed survivorship. Biotropica, 22(4), 423-424.
Figueira, C.E.J., Vasconcello-Neto, J. & Telxeira De Souza, A.L. (1994). Saurocory in Melocactus violaceus (Cactaceae). Biotropica, 26(3), 295-301.
Fischer, S.F., Poschlod, P., & Beinlich, B. (1996). Experimental Studies on the dispersal of plants and animals on sheep in calcareous grasslands. Journal of Applied Ecology, 33, 1206–22.
Hendrix, L.B., & Smith, S. D. (1986). Post-eruption revegetation of Isla Fernandina, Galapagos (Ecuador). II. National Geographic Research 2, 6-16.
Iverson, J.B. (1985). Lizards as seed dispersers? Journal of Herpetology, 19(2),
-293.
Levin, S.A., Muller-Landau, H., Nathan, R. & Chave, J. (2003). The ecology and evolution of seed dispersal: a theoretical perspective. Annual Review of Ecology and Systematics, 34, 575-604.
Manzano, P. & Malo, J.E. (2006) Extreme long-distance seed dispersal via sheep. Frontiers in Ecology and the Environment, 4, 244–248.
Moll, D., & Jansen P. K. (1995). Evidence for a role in seed dispersal by two tropical herbivorous turtles. Biotropica, 27,121-127.
Nassar, J. M. & Ramírez, N. (2004). Reproductive biology of the melon cactus, Melocactus curvispinus (Cactaceae). Plant Systematica and Evolution, 248(1-4), 31-44.
Nogales, M., Heleno, R., Traveset, A. & Vargas, P. (2012). Evidence for overlooked mechanisms of long-distance seed dispersal to and between oceanic islands. New Phytologist, 194, 313–317.
Olensen, J.M. & Valido, A. (2003). Lizards as pollinators and seed dispersers: an
island phenomenon. Trends in Ecology and Evolution, 18(4), 177-181.
Rick, C.M. & Bowman, R.I. (1961) Galápagos tomatoes and tortoises. Evolution, 15, 407–417.
Rojas-Aréchiga, M. &Vázquez-Yanes, C. (2000). Cactus seed germination: a review. Journal of Arid Environments, 44, 85–104.
Ruiz, A, Santos M, Cavelier, J. & Soriano, P.J. (2000) Estudio Fenológico de Cactaceas en el Enclave Seco de la Tatacoa, Colombia. Biotropica, 32, 397-407.
Rust, R.W. & Roth, R. R. (1981). Seed production and seedling establishment in the may apple, Podophyllum peltatum L. American Midland Naturalist, 105, 51-60.
Sarukhan, J., Martínez-Ramos, M. & Pinero, D. (1984). The analysis of demographic variability at the individual levels and its population conse- quences. In: Dirzo, R. & Sarukhan, J. (eds.), Perspectives in Plant Population Ecology, pp. 83-106. Sinauer Associates, Inc., Sunderland, MA.
Stiles, E.W. (2000) Animals as seed dispersers. In: Fenner, M (ed.), Seeds: The Ecology of Regeneration in Plant Communities, pp. 111–124. CAB International, Wallingford, UK.
Tiffney, B.H. (1984) Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Annals of the Misssouri Botanical Garden, 71, 55- 576.
Traveset, A. (1990a). Ctenosaura similis Gray (Iguanidae) as a seed disperser in a Central American deciduous forest. American Midland Naturalist, 123, 402-404.
Traveset, A. (1990b) Post-dispersal predation of Acacia farnesiana seeds by Stator vachelliae (Bruchidae) in Central America. Oecologia, 84, 506–512.
Valido, A., Nogales, M. & Medina, F.M. (2003). Fleshy fruits in the diet of Canarian lizards Gallotia galloti (Lacertidae) in a xeric habitat of the Island of Tenerife. Journal of Herpetology, 37(4), 741-747.
Valido, A., & Nogales, M. (1994). Frugivory and seed dispersal by the lizard Gallotia galloti (Lacertidae) in a xeric habitat of the Canary Islands. Oikos, 70, 403-411.
Varela, R.O. & Bucher, E.H. (2002). The lizard Teius teyou (Squamata: Teiidae) as a legitimate seed disperser in the dry chaco forest of Argentina. Studies on Neotropical Fauna and Environment, 37(2), 115-117.
Wotton, D.M. (2002). Effectiveness of the common gecko (Hoplodactylus maculatus) as a seed disperser on Mana Island, New Zealand. New Zealand Journal of Botany, 40, 639-647.
Whitaker, R. D. (1987). The roles of lizards in New Zealand plant reproductive strategies. New Zealand Journal of Botany, 25, 315-328.
Willson, M.F., Sabag, C., Figueroa, J., Armesto, J.J. & Caviedes, M. (1996) Seed dispersal by lizards in Chilean rainforest. Revista Chilena de Historia Natural, 69, 339–342.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
 |
Lasso, E. & Barrientos, L. (2015). Epizoochory in dry forest green iguana: an overlooked seed dispersal mechanism?. Colombia Forestal, 18(1), 151-159 |
Artículo de investigación
EPIZOOCHORY IN DRY FOREST GREEN IGUANA: AN OVERLOOKED SEED DISPERSAL MECHANISM?
Epizoocoría por medio de iguanas en el bosque seco: ¿un mecanismo de dispersión de semillas pasado por alto?
Eloisa Lasso1 & Lucas Santiago Barrientos2
1Departamento de Ciencias Biológicas, Universidad de los Andes, Bogotá, Colombia. Smithsonian Tropical Research Institute, Apt. 0843-03092, Balboa, Ancón, Panamá. e.lasso@uniandes.edu.co. Autor para correspondencia.
2Departamento de Ciencias Biológicas, Universidad de los Andes, Bogotá, Colombia. ls.barrientos50@uniandes.edu.co
Recepción: 28 de septiembre de 2014 / Aprobación: 25 de noviembre de 2014
ABSTRACT
The role of animals as seed dispersal vectors, including dispersal by reptiles (saurochory), is widely acknowledged. Most reports of saurochory have been via endozoochory through feces deposition. We present the first evidence of epizoochory in Iguana iguana from Tatacoa dry forest in Colombia via seeds attached to the snout. Our results show that seeds of Melocactus curvispinus ingested by iguana suffer from their passage through the digestive tract while seeds transported while attached to the snout germinate faster and in higher numbers. Our data suggest that we may have overlooked an alternative means of seed dispersal by lizards that do not comprise a passage through their digestive tract, and that deserves further attention for the understanding of dry forest ecology.
Key words: epizoochory, Iguana iguana, Melocactus curvispinus, saurochory, Tatacoa, tropical dry forest.
RESUMEN
El papel que juegan los animales como vectores en la dispersión de semillas es extensamente reconocido, incluso la dispersión por reptiles, mejor conocida como saurocoría. La mayoría de los reportes de saurocoria han sido a través de endozoocoria, es decir, el transporte interno de semillas que han sido ingeridas y luego depositadas con las heces. Presentamos la primera evidencia de epizoocoría en Iguana iguana por medio del transporte externo de semillas adheridas a su hocico observado en el bosque seco de Tatacoa, Colombia. Nuestros resultados muestran que las semillas del cactus Melocactus curvispinus ingeridos son probablemente dañadas al pasar por el tracto digestivo de la iguana, mientras que las semillas transportadas externamente germinan más rápido y en mayor número. Nuestros datos sugieren que es posible que hayamos estado ignorando un mecanismo alternativo de dispersión de semillas por lagartos que no comprende el paso a través del tracto digestivo, lo cual merece mayor atención para una mejor comprensión de la ecología del bosque seco.
Palabras clave: epizoocoria, Iguana iguana, Melocactus curvispinus, saurocoria, Tatacoa, bosque seco tropical
INTRODUCTION
Saurochory, or seed dispersal by reptiles such as lizards, snakes, and tortoise is an ancient form of seed dispersal. Reptiles played an important role in the reproduction of plants in the Jurassic (Howe & Westley, 1988) and are recognized as important seed dispersers of the first gymnosperms and angiosperms (Tiffney, 1984). Even today, in a world where the literature on seed dispersal is dominated by birds and mammals, it is generally recognized that fruits are an important part of the diet of many reptiles (Iverson, 1985; Fialho, 1990; Benítez-Malvido et al., 2003; Valido et al., 2003) and that some plants depend on reptiles for dispersal (Rick & Bowman, 1961; Wotton, 2002; Celedón-Neghme et al., 2008) especially on Islands (Olensen & Valido, 2003) and arid environments (Valido et al., 2003) where lizards can be the dominant vertebrate herbivores. Even though fruit eating has been reported for 202 lizard species in 19 families (Olensen & Valido, 2003), and several studies show that lizards can be important seed dispersers, the scientific community stills regards lizard-plant interactions as rare and unimportant for the understanding of ecosystem functioning and to the evolution of fruit traits.
A key and still inconclusive aspect of the lizard-plant mutualistic interaction that needs to be clarified is whether lizards are acting as seed predators or seed dispersers (Iverson, 1985), including the question of whether seed passage through the reptile's digestive tract is critical for breaking seed dormancy (Figueira et al., 1994) or if seeds eaten by reptiles lose their viability or are harmed while passing through the digestive tract. Results to date have been somewhat contradictory, but the majority of studies show that seeds that have passed through reptile guts remain viable (or at least some of them) and often show enhanced germination (Rust & Roth, 1981; Iverson, 1985; Willson et al., 1996; Castilla, 1999; Benítez-Malvido et al., 2003). Evidence from histological cuts of seeds ingested by lizards indicates that abrasion of the seed coats increases permeability to water and gases facilitating germination (Celedón-Neghme et al., 2008). However, depending on the species, this abrasive process could damage the seed as suggested by the finding of some authors that report that ingestion by lizards can negatively affects seed germination (Traveset, 1990b; Valido & Nogales, 1994; see Traveset 1998 for a review; Castilla, 1999; Varela & Bucher, 2002). To date what remains clear from the literature on saurochory is that the effect produced on seeds by passage through lizard guts varies among plant species, sometimes being positive, sometimes negative, and some times neutral. None of these studies, however, have considered the possibility of external passive seed transportation or epizoochory on lizards.
Most literature reviews on animals as vectors for seed movement recognize that a large number of seeds are moved by passive attachment to the fur of mammals and feathers of birds (Stiles, 2000; Couvreur, Verheyen & Hermy, 2005) but there is no mention of epizoochory by reptiles. Recently Burgin & Renshaw (2008) reported epizoochory by a fresh water turtle via their carapacial algal mats. For lizards, alternative mechanisms of dispersal to seed transport in the digestive tract and deposition in feces has not been considered. None of those studies addressing lizard-plant interactions have ever evaluated the state of seeds that remain stuck to the lizard snout after they eat the fruits, which could be safely transported away from the parent plant and still remain viable. In this study we report our results on germination time and success from seeds collected from the external area of the snout (Figure 1) and from the feces of an individual of Iguana iguana (Iguanidae) found feeding on Melocactus curvispinus (Cactaceae) at the Tatacoa very dry forest on Central Colombia. We compare these data with germination success of seeds collected directly from ripe fruits extracted in the field.
MATERIALS AND METHODS
Study Area
The study was conducted at Tatacoa dry forest (3°17'N-74°58'O; 580m of altitude) located in the Northern part of the Colombian state of Huila, at the east side of the Magdalena river (Ruiz et al., 2000). Tatacoa is the second largest arid zone in Colombia with 330 km2 of land. Mean annual precipitation is < 1 000 mm with two rainy periods one from March to May and other from October to November (Ruiz et al., 2000). Mean temperature is 28°C but can reach 40°C in the dry months. The original vegetation of the area corresponds to a tropical dry forest according to Holdridge life zones; however, the natural vegetation has been drastically altered because of extensive livestock farming (cattle initially and finally goat), which resulted in severe erosion (Cavelier et al., 1996). Typical dry forest tree species can still be found along rivers such as Pithecellobium dulce, Acacia farnesiana, Prosopis juliflora, Anacardium excelsum, Ceiba pentandra, Ficus dendrocida, Pseudobombax sp; far from rivers the landscape is dominated by several species of cacti including Opuntia, Acanthocereus, Stenocereus and Melocactus intermingled with some shrubs species like Randia aculeata (Figueroa & Galeano, 2007).
One of the characteristic plant species of the study area is Melocactus curvispinus, which is a small globose cactus. This species has an extended geographical distribution from Mexico to northern South America, including parts of the Caribbean, where it is found in the lowlands up to 1 250 of altitude, always associated with arid and semiarid environments. M. curvispinus has open bright-colored flowers and bright red conical fleshy berries embedded on the cephalium, a structure made of white woolly fibers that probably protect the flowers and fruits from the high temperatures and dry conditions of their habitat. Saurochory has been reported for Melocactus violaceus from Brazil where seed passage through the lizard digestive tract is necessary to break seed dormancy (Figueira et al., 1994) and for Melocactus schatzlii in Venezuela (Casado & Soriano, 2010). In Tatacoa, fruits of M. curvispinus can be eaten by birds, ants, lizards (personal observation) and foxes (Ruiz et al., 2000) but is not known whether their seeds need special treatment to break dormancy and germinate as was shown for M. violaceus from Brazil.
In October 2013 we observed a juvenile I. iguana feeding on Melocactus fruits and noticed plenty of seeds attached to its snout area (Figure 1). We captured the Iguana and collected the seeds from the snout area and saved on vials for germination trials. We kept the iguana under captivity to collect its feces and seeds that passed through its digestive tract. Simultaneously, we also collected ripe fruits from the plant where the Iguana was feeding and from 3 more nearby plants. All seeds were taken to the lab and surface sterilized with a 10% Clorox solution, then washed with distilled water and sown to germinate under controlled conditions (mean temperature 28°C, 40% RH, 12h light/12h dark regime) for 40 days in Petri dishes using cotton layers as substrate. We arranged seeds into groups of 15 seeds per Petri dish. We had 30 seeds from the snout, 60 from the feces and 60 from 5 ripe fruits collected directly from four plants. To ensure similar environmental conditions for all seeds, Petri dishes were placed in a controlled room under lamps that provided 300 µmole photons/m2/s of light intensity, and were rotated haphazardly every week. Seeds were observed every 4 days for 40 days and were registered as germinated when we observed that the radicle extruded from the seed coat.
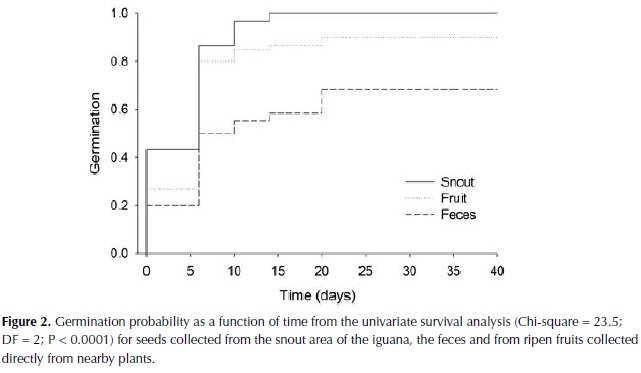
Statistical analysis. To evaluate whether seeds that pass though the digestive tract of the iguana germinate faster or slower than seeds transported on the snout and to determine how their germination compares with seeds collected directly from the fruit we did an univariate survival analysis computing product-limit (Kaplan-Meier) estimates. For this analysis we analyzed the fate of 150 seeds (30 snout seeds, 60 feces seeds and 60 fruit seeds) from day 1 to day 40. We registered the date when the seed was observed germinating and if a seed didn't germinate during the 40 days, we censored those observations. From this analysis we estimated the probability of germination as a function of time and the standard deviation adjusted for censoring for each group of seeds. To test for homogeneity across groups we computed the Log Rank and the generalized Wilcoxon Chi-square statistics, which gave similar results, and we only present Log-Rank results. The germination time is expressed as the time in which 50% of the seeds germinated (T50). To assess differences in percentage seed germination for seeds from fruits and from lizard-ingested and lizard-snout treatments we performed an Analysis of Variance after normalizing data using an arcsine function (Zar, 1996). All analyzes were done using JMP 10.0.2 (SAS Institute, Cary, NC).
RESULTS
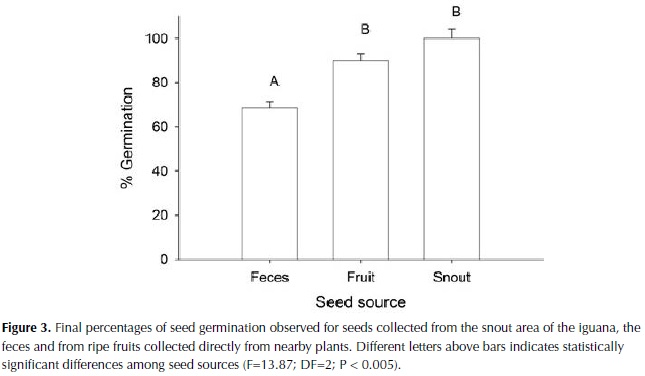
We found significant differences in germination time among seeds collected from the iguana feces, the snout area and directly from the fruits (Chi-square = 23.5; DF = 2; P < 0.0001; Figure 2). Seeds collected from the snout germinated faster (T50 = 9.0 + 0.6 days) than seeds collected from the feces (T50 = 20.15 + 1.7 days), while seeds collected directly from fruit had intermediate values (T50 = 12.6 + 1.2 days). We also found that seed viability, represented as the percentage of seeds that were able to germinate, was negatively affected by the passage through the iguana guts (F=13.87; DF=2; P < 0.005; Figure 3). Only 67% of the seeds collected from feces germinated in comparison to 100% for seeds collected from the snout and 90% for seeds collected from fruits.
DISCUSSION
Our results show that M. curvispinus may suffer and gain from the interaction with iguanas as potential seeds dispersers depending on whether seeds are transported externally (epizoochory) or ingested, transported, and defecated (endozoochory). The comparison of the fate of the ingested and non-ingested seeds indicates that ingestion by lizards adversely affects Melocactus recruitment, as other authors have reported for other plant species dispersed by lizards (Traveset, 1990b; Valido & Nogales, 1994; Willson et al., 1996; Castilla, 1999; Varela & Bucher, 2002). However, iguanas seem to be able to also transport seeds attached to their body, which remain stuck to the lizard snout after they eat the fruits, keeping their viability and germinating in higher numbers and faster than those ingested. How far seeds can be transported by such means needs to be evaluated. Seeds are not loosely attached, as we actually needed to exert force to pull them out of the snout using tweezers, which will possibly enhance their chance to be transported away from the parent plant as expected for a "good frugivorous disperser".
Seeds transported on the iguana snout will probably have higher recruitment success given that they germinate faster than seeds ingested. The time required for germination and seedling establishment is crucial in increasing establishment success (Bazzaz et al., 1982). Evidence suggests that seeds that germinate faster produce more vigorous seedlings with greater probabilities of survival than late recruitments of the same seedling cohort (Sarukhan et al., 1984) and are also more likely to avoid predators and pathogens (Andresen, 1999). In dry forest habitats characterized by short rainy periods suitable for seed germination and seedling recruitment, the slower germination rates observed for seeds ingested by iguana could seriously hinder establishment success of Melocactus if they take longer to develop a root system that can reach deeper moist soil layers before water scarcity arrives.
Cactaceae are one of the best-represented families in arid and semi-arid regions (Rojas-Aréchiga & Vázquez-Yanes, 2000). In Tatacoa there are 8 species of cactus belonging to seven genera (Figueroa & Galeano, 2007) and M. curvispinus is one of the most abundant. They are adapted to the harsh condition of this degraded dry forest being tolerant to water scarcity, high temperatures and poor soils. The bright colored fleshy fruits are produced all year long (Nassar & Ramírez, 2004) representing an important source of sugar and water for many animals in this harsh environment. Animals definitively benefits from the interaction but Melocactus might benefit or suffer from the interaction, depending on the animal and the way seeds are transported. Most seeds that are dispersed via animals are characterized by a very thick or resistant testa, which can withstand stomach acids and enzymes. Seeds of some species, like Melocactus violaceus, which grows in the open sandy soils in the southern Brazilian coastal area, can only break dormancy when they pass through the lizard digestive tract (Figueira et al., 1994). The abrasive process of the seed coat seems to be necessary to increase permeability to water and gases facilitating germination (Celedón-Neghme et al., 2008). Likewise, seeds of Melocactus schatzlii from the Lagunillas semiarid enclave in Mérida-Venezuela are thick enough to withstand the passage through lizard digestive tract since defecated seeds germinated in similar numbers as non-defecated seeds (Casado & Soriano 2010). Nevertheless, our results suggest that the testa of M. curvispinus seeds is perhaps too thin and seeds are damaged while passing through the digestive tract of the iguana. What remains clear by comparing these three Melocactus species is that the effect produced on seeds by passage through lizard guts varies among plant species, even of congeneric species.
Most literature reviews on animals as vectors for seed movement acknowledge that seeds can be transported attached to the fur of mammals and feathers of birds (Stiles, 2000; Couvreur, Verheyen & Hermy, 2005). So far, epizoochory on reptiles has only being reported for a fresh water turtle, where seeds stick to the algal mats attached to the turtle's carapace (Burgin & Renshaw, 2008). Our report is the first describing epizoochory for an iguana species in a tropical dry forest and discusses an alternative means of dispersal that needs to be evaluated in more detail. Other studies show that, although not as mobile as birds or mammals, reptiles are important dispersal vectors in arid and island ecosystems (Whitaker, 1987; Traveset, 1990a; Valido & Nogales, 1994; Moll & Jansen, 1995). Iguanas in particular might play a significant role in the reproductive strategies of a number of tropical dry forest plants. The Black iguana Ctenosaura similis is one of the principal dispersers of the tree Acacia farnesiana and is probably an important seed disperser in the tropical dry forest of Costa Rica (Traveset, 1990a,b). In a Mexican dry forest, individuals of I. iguana consumed seeds of Cordia alba, Momordica charantia, Pithecellobium dulce, and Lycopersicon esculentum), and those seeds ingested by the iguana had higher rate of germination (Benítez-Malvido et al., 2003). In the Galapagos Islands, iguanas appear to be fundamental for vegetation recovery as they dispersed seeds of many plant species that are important for regeneration (Hendrix & Smith, 1986). The role of iguanas as dispersal vectors in Tatacoa dry forest needs to be evaluated further, given that this forest is severely degraded and iguanas could be fundamental for forest regeneration.
Seasonally dry tropical forests are widely agreed to be the most endangered tropical forest type in the world and their conservation will depend on better basic biological knowledge about the ecological processes that govern the dry forest ecosystem. Here we present a new observation on seed ecology that involves an overlooked dispersal mechanism through epizoochory in iguanas that do not comprise a passage through their digestive tract. Epizoochory is a largely neglected aspect of seed ecology (Levin et al., 2003) in spite of the importance of this external seed transportation, which can be one of the most effective long-distance dispersal mechanisms (Manzano & Malo, 2006; Bullock et al., 2011; Nogales et al., 2012; Fischer et al., 1996). The external passive seed transportation of non-damaged seeds by the iguanas of Tatacoa deserve further attention to evaluate how many of those seeds can be incorporated into the soil seed bank and can germinate if they are deposited onto a safe microsite where conditions are suitable.
CONCLUSION
The green iguana I. iguana disperses seeds of M. curvispinus at Tatacoa dry forest (Huila, Colombia) both by endozoochory (internal transport of seeds after ingestion) and by epizoochory (external transport of seeds stuck to the snout). Seeds transported externally germinate in higher numbers and faster than seeds ingested and defecated, suggesting that iguanas could be considered mild seed predator and seed disperser simultaneously depending on the mean of seed transportation. This is the first report of epizoochory by iguanas, an overlooked and alternative means of seed dispersal by lizards that does not comprise a passage through their digestive tract, which deserves further attention for the understanding of dry forest ecology.
ACKNOWLEDGMENTES
We would like to thank all students of the plant physiology course 2014-1 from Universidad de los Andes that help us follow and capture the iguana. We also thank James Dalling for reviewing and commenting on this manuscript. Funding for this research comes from Eloisa Lasso's FAPA funds P12.160422.001 from Universidad de los Andes.
BIBLIOGRAFIC REFERENCES
Andresen, E. (1999). Seed dispersal by monkeys and the fate of dispersed seeds in a Peruvian rain forest. Biotropica, 31,145-158.
Bazzaz, F. A., Levin, D. A. & Schmierbach, M. R. (1982). Differential survival of genetic variants in crowded populations of Phlox. Journal of Applied Ecology, 19, 891-900.
Benítez-Malvido, J., Tapia, E. Suazo, I., Villaseñor, E. & Alvarado, J. (2003). Germination and seed damage in tropical dry forest plants ingested by Iguanas. Journal of Herpetology,37(2), 301-308.
Bullock, J.M., Galsworthy, S.J., Manzano, P., Poschlod, P., Eichberg, C., Walker, K. & Wichmann, M.C. (2011). Process-based functions for seed retention on animals: a test of improved descriptions of dispersal using multiple data sets. Oikos, 120, 1201-1208.
Burgin, S. & Renshaw, A. (2008). Epizoochory, algae and the Australian Eastern Long-Necked turtle Chelodina longicollis (Shaw). The American Midland Naturalist, 160(1), 61-68.
Casado, R. & Soriano, P. (2010). Fructificación, frugivoría y dispersión en el cactus globular Melocactus schatzlii en el enclave semiarido de Lagunilla, Mérida, Venezuela. Ecotrópicos, 23(1), 18-36.
Castilla, A.M. (1999). Podarcis lilfordi from the Balearic islands as a potential disperser of the rare Mediterranean plant Withania frutescens. Acta Oecologica, 20(2), 103-107.
Cavelier, J., Ruiz, A., Santos, M. Quiñones, M. & Soriano, P. (1996). El proceso de degradación y sabanización del Valle Alto del Magdalena. Informe inédito. Bogotá: Fundación del Alto Magdalena.
Celedón-Neghme, C. L. San Martin, A., Victoriano, P. F., &Cavieres, L. A. (2008). Legitimate seed dispersal by lizards in an alpine habitat: The case of Berberis empetrifolia (Berberidaceae) dispersed by Liolaemus belii (Tropiduridae). Acta Oecologica, 33, 265-271.
Couvreur, M., Verheyen, K. & Hermy, M. (2005). Experimental assessment of plant seed retention times in fur of cattle and horse. Flora, 200, 136-147.
Fialho, R.F. (1990). Seed dispersal by a lizard and a treefrog effect of dispersal site on seed survivorship. Biotropica, 22(4), 423-424.
Figueira, C.E.J., Vasconcello-Neto, J. & Telxeira De Souza, A.L. (1994). Saurocory in Melocactus violaceus (Cactaceae). Biotropica, 26(3), 295-301.
Figueroa, Y.C. & Galeano, G. (2007). Lista comentada de las plantas vasculares del enclave seco interandino de la Tatacoa (Huila, Colombia). Caldasia, 29(2), 263-281.
Fischer, S.F., Poschlod, P., & Beinlich, B. (1996). Experimental studies on the dispersal of plants and animals on sheep in calcareous grasslands. Journal of Applied Ecology,33, 1206-22.
Hendrix, L.B., & Smith, S. D. (1986). Post-eruption revegetation of Isla Fernandina, Galapagos (Ecuador). II. National Geographic Research, 2, 6-16.
Howe, H.F., & Westley, L.C. (1988). Ecological relationships of plants and animals. New York: Oxford University Press.
Iverson, J.B. (1985). Lizards as seed dispersers? Journal of Herpetology,19(2), 292-293.
Levin, S.A., Muller-Landau, H., Nathan, R. & Chave, J. (2003). The ecology and evolution of seed dispersal: a theoretical perspective. Annual Review of Ecology and Systematics,34, 575-604.
Manzano, P. & Malo, J.E. (2006). Extreme long-distance seed dispersal via sheep. Frontiers in Ecology and the Environment, 4, 244-248.
Moll, D., & Jansen P. K. (1995). Evidence for a role in seed dispersal by two tropical herbivorous turtles. Biotropica, 27, 121-127.
Nassar, J. M. & Ramírez, N. (2004). Reproductive biology of the melon cactus, Melocactus curvispinus (Cactaceae). Plant Systematics and Evolution, 248(1-4), 31-44.
Nogales, M., Heleno, R., Traveset, A. & Vargas, P. (2012). Evidence for overlooked mechanisms of long-distance seed dispersal to and between oceanic islands. New Phytologist, 194, 313-317.
Olensen, J.M. & Valido, A. (2003). Lizards as pollinators and seed dispersers: an island phenomenon. Trends in Ecology and Evolution,18(4), 177-181.
Rick, C.M. & Bowman, R.I. (1961). Galápagos tomatoes and tortoises. Evolution, 15, 407-417.
Rojas-Aréchiga, M. &Vázquez-Yanes, C. (2000). Cactus seed germination: a review. Journal of Arid Environments,44, 85-104.
Ruiz, A, Santos M, Cavelier, J. & Soriano, P.J. (2000). Estudio Fenológico de Cactaceas en el Enclave Seco de la Tatacoa, Colombia. Biotropica, 32, 397-407.
Rust, R.W. & Roth, R. R. (1981). Seed production and seedling establishment in the may apple, Podophyllum peltatum L. American Midland Naturalist, 105, 51-60.
Sarukhan, J., Martínez-Ramos, M. & Pinero, D. (1984). The analysis of demographic variability at the individual levels and its population consequences. In R. Dirzo & Sarukhan, J. (eds.). Perspectives in Plant Population Ecology (pp. 83-106). MA-Sunderland: Sinauer Associates, Inc.
Stiles, E.W. (2000). Animals as seed dispersers. In M. Fenner (ed.).Seeds: The Ecology of Regeneration in Plant Communities(pp. 111-124). UK-Wallingford: CAB International.
Tiffney, B.H. (1984). Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Annals of the Misssouri Botanical Garden, 71, 55-576.
Traveset, A. (1990a). Ctenosaura similis Gray (Iguanidae) as a seed disperser in a Central American deciduous forest. American Midland Naturalist, 123, 402-404.
Traveset, A. (1990b). Post-dispersal predation of Acacia farnesiana seeds by Stator vachelliae (Bruchidae)in Central America. Oecologia, 84, 506-512.
Traveset, A. (1998). Effect of seed passage through vertebrate frugivores'guts on germination: a review. Perspectives in Plant Ecology, Evolution and Systematics, 1/2, 151-190.
Valido, A., & Nogales, M. (1994). Frugivory and seed dispersal by the lizard Gallotia galloti (Lacertidae) in a xeric habitat of the Canary Islands. Oikos, 70, 403-411.
Valido, A., Nogales, M. & Medina, F.M. (2003). Fleshy fruits in the diet of Canarian lizards Gallotia galloti (Lacertidae) in a xeric habitat of the Island of Tenerife. Journal of Herpetology, 37(4), 741-747.
Varela, R.O. & Bucher, E.H. (2002). The lizard Teius teyou (Squamata: Teiidae) as a legitimate seed disperser in the dry chaco forest of Argentina. Studies on Neotropical Fauna and Environment,37(2), 115-117.
Whitaker, R. D. (1987). The roles of lizards in New Zealand plant reproductive strategies. New Zealand Journal of Botany, 25, 315-328.
Willson, M.F., Sabag, C., Figueroa, J., Armesto, J.J. & Caviedes, M. (1996). Seed dispersal by lizards in Chilean rainforest. Revista Chilena de Historia Natural, 69, 339-342.
Wotton, D.M. (2002). Effectiveness of the common gecko (Hoplodactylus maculatus) as a seed disperser on Mana Island, New Zealand. New Zealand Journal of Botany,40, 639-647.
Zar, J.H. (1996). Biostatistical Analysis. N.J-Eryelwood Cliffs: Prentice-Hall.
License
Colombia Forestal retains the patrimonial rights (copyright) of the published works, and favors and allows the reuse of the same under the Creative Commons Attribution-ShareAlike 4.0 International license, so they can be copied, used, disseminated, transmitted and exhibited publicly, provided that:
You acknowledge the credits of the work in the manner specified by the author or licensor (but not in a way that suggests that you have their support or that they endorse your use of their work).