DOI:
https://doi.org/10.14483/23448393.13418Published:
2018-09-28Issue:
Vol. 23 No. 3 (2018): September - DecemberSection:
Environmental EngineeringAdsorción de Cadmio, Cobre y Plomo en Bentonita, Caolín y Zeolita Naturales y Modificadas: Una Revisión de los Parámetros de Operación, Isotermas y Cinética
Cadmium, Copper and Lead Adsorption on Natural and Modified Bentonite, Kaolin and Zeolite: A Review of Process Parameters, Isotherms and Kinetics
Downloads
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Recibido: 4 de junio de 2018; Revisión recibida: 24 de agosto de 2018; Aceptado: 18 de septiembre de 2018
Resumen
Contexto:
La adsorción es un proceso efectivo para el tratamiento de aguas residuales contaminadas con metales pesados. El uso de adsorbentes de bajo costo incrementa la ventaja y competitividad de este proceso; en este sentido, arcillas y zeolitas, naturales y modificadas, han sido usadas ampliamente para la remoción de metales pesados de aguas residuales.
Método:
Se revisaron, analizaron y compararon los estudios realizados durante la última década referentes a la adsorción de cadmio, cobre y plomo por bentonita, caolín y zeolita, naturales y modificadas.
Resultados:
Se analizan diferentes parámetros de operación, condiciones de equilibrio y cinética. Los parámetros de operación estudiados son la concentración inicial de metales, pH de la solución, dosis de adsorbente y temperatura del sistema. Se presenta una recopilación de la eficiencia de los sistemas respecto a la capacidad máxima de adsorción; además, se discuten los modelos de isotermas usados para analizar el equilibrio de adsorción, así como los modelos cinéticos y de difusión en los estudios revisados.
Conclusiones:
El uso de bentonita, caolín y zeolita es efectivo para la remoción de cadmio, cobre y plomo de soluciones acuosas. La influencia de diversos parámetros de operación se ve reflejado en la variabilidad de las capacidades de adsorción de cadmio, cobre y plomo. En relación con el equilibrio de adsorción en la mayoría de estudios revisados, la isoterma de Langmuir es la que mejor se ajusta a los datos; en cuanto a la cinética, el modelo de pseudosegundo orden es el que mejor se ajusta a los datos.
Palabras clave:
Adsorbentes naturales, arcillas, cinética de adsorción, clinoptilolita, equilibrio de adsorción, metales pesados, parámetros de operación..Abstract
Context:
Adsorption is a tertiary wastewater treatment that can be effectively employed to remove metal ions from aqueous solutions. Natural and modified clays and zeolites have been widely use as low-cost materials to increase the competitive advantage of the process.
Method:
A comprehensive review was made amongst articles, that during the last decade, have studied cadmium, copper and lead adsorption onto natural and modified bentonite, clay and zeolite.
Results:
Different process parameters, equilibrium conditions and kinetics were analyzed. Operation parameters studied were initial metal ion concentration, solution pH, adsorbent dosage and temperature. Compilation of system efficiencies, in regards to maximum adsorption capacity, is presented. Isotherm models to assess adsorption equilibrium as well as kinetic and diffusion models in studies reviewed are discussed.
Conclusions:
Bentonite, kaolin and zeolite have been proven to be adequate materials to remove cadmium, copper and lead from aqueous solutions. The different adsorption capacities of cadmium, lead and copper are a reflection of the influence of many process parameters. The Langmuir isotherm usually describes best the equilibrium adsorption in the articles reviewed. Finally, the pseudo-second orden model better describes the kinetics in many cases.
Keywords:
Clays, clinoptilolite, natural adsorbents, heavy metals, process parameters, adsorption equilibrium, kinetics adsorption..Introducción
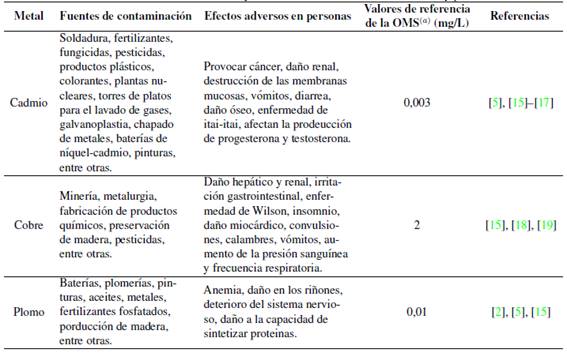
En la actualidad, la intensa actividad industrial produce aguas residuales que contienen diversos metales pesados, entre ellos el cadmio, cobre y plomo; así, la contaminación de los ecosistemas acuáticos, resultante del vertimiento de esta agua sin tratar, es una de las principales preocupaciones de los gestores ambientales debido la alta toxicidad y persistencia. Según la EPA (siglas en inglés de Agencia de Protección Ambiental de Estados Unidos), varios metales pesados figuran en lista de contaminantes prioritarios por ser una preocupación para la salud pública, estos son: arsénico, cromo, cobalto, níquel, cobre, zinc, plata, cadmio, mercurio, talio, titanio, selenio y plomo [1]. El cadmio, cobre y plomo provienen de numerosas actividades y, por encima de los límites permisibles, ocasionan efectos adversos y desórdenes en seres humanos (Tabla I) al acumularse en tejidos vivos [2].
(a)Valores referenciales sugeridos por la Organización Mundial de la Salud (OMS) cuya presencia en el agua de consumo puede afectar la salud.
Tabla I: Fuentes, efectos adversos y valores referenciales del cadmio, cobre y plomo.
Durante los últimos años, el proceso de adsorción ha tomado importancia como un método sencillo y económico para la remoción de contaminantes en el tratamiento de aguas [3], lo anterior debido a la flexibilidad en el diseño, la calidad del efluente tratado, la reversibilidad del proceso y la posibilidad de regenerar el adsorbente empleado [4]. Entre los adsorbentes más económicos y eficientes se encuentran materiales naturales como la bentonita, el caolín y las zeolitas, cuya estructura y composición les confiere una gran capacidad de intercambio catatónico y los hace materiales ideales para la remoción de diferentes tipos de contaminantes [5]. Los tratamientos físicos y químicos han sido usados con ´éxito para aumentar la superficie específica de los minerales y, en consecuencia, la capacidad de adsorción [2], [6].
En la última década se han hecho artículos de revisión que abordan la adsorción de contaminantes industriales en soluciones acuosas empleando arcillas, zeolita [7] y bentonita [8], [9], algunas recopilaciones se han enfocado en la adsorción de metales pesados con arcillas [10], [11] y otros minerales incluyendo zeolita [12], [13]; sin embargo, a pesar de que algunos autores han recopilado información sobre la adsorción de zinc [14] y níquel [12], no se han hecho revisiones sobre el cadmio, cobre y plomo. Por esta razón el objetivo de este trabajo es analizar los parámetros más importantes que influyen en el proceso de adsorción de cadmio, cobre y plomo empleando bentonita, caolín y zeolita; asimismo, se resumirán los principales hallazgos en cuanto al equilibrio y cinética de adsorción.
Para la presente revisión se han tomado en cuenta artículos científicos que hayan usado bentonita, caolín y zeolita para la adsorción de cadmio, cobre o plomo. Se han considerado estudios a partir del 2010.
Clasificación de bentonita, caolín y zeolita
Las arcillas son uno de los materiales de mayor abundancia sobre la tierra y, debido a la capacidad catalítica de neutralizar elementos químicos contaminantes, han sido usados en la protección ambiental para la disposición y almacenamiento de químicos peligrosos. Como barrera para el almacenamiento de sustancias peligrosas, una de las características más importantes de las arcillas esa capacidad de adsorción [5].
Las arcillas pertenecen a la familia de los aluminosilicatos que se distinguen por tener estructuras en capas compuestas por láminas de polímeros tetraédricos de SiO4 unidos a láminas octaédricas (Al, Mg, Fe)(O,OH)6 [5]. Las zeolitas naturales son químicamente similares a las arcillas, sus moléculas se encuentran conectadas en una estructura enmarcada caracterizada por espacios entre los grupos moleculares, mientras que las arcillas se caracterizan por capas tetraédricas y octaédricas apiladas de determinadas maneras, lo que origina capas ligeramente conectadas [5].
La clasificación de Grim coloca a las caolinitas en el grupo de dos capas (1:1, una capa de tetraedros de sílice y otra capa de octaedros de alúmina), mientras que a las bentonitas en el grupo de tres capas (2:1, dos capas de tetraedros de sílice y una capa central dioctaédrica o trioctaédrica). Las zeolitas pertenecen al grupo mineral de los tectosilicatos, poseen tres estructuras tridimensionales de silicato y alúmina unidas entre sí por átomos de oxígeno compartidos [12].
Mecanismos de adsorción
La adsorción es una operación de transferencia de masa muy usada en la práctica para remover sustancias de fluidos (gases o líquidos), de manera general se puede definir como el enriquecimiento de especies químicas de un fluido en la superficie de un líquido o sólido. Las ventajas de emplear arcillas y zeolitas como adsorbentes para la remoción de metales pesados son la elevada capacidad de adsorción e intercambio de iones, baja permeabilidad, capacidad de hinchamiento, estabilidad química y física y elevada área superficial [5].
La adsorción de metales pesados es un proceso complejo que refleja la tendencia de las arcillas de formar enlaces covalentes. El grado de remoción de metales no es solo una función de la capacidad de intercambio catatónico de las arcillas, la adsorción de iones implica varios procesos que incluyen la formación de complejos superficiales (los cuales pueden ser directos de esfera interna o indirectos de esfera externa), intercambio de iones y precipitación superficial. La formación de complejos superficiales, que es parte de la adsorción específica, ocurre en los bordes de la estructura laminar de las arcillas, implica la formación de enlaces directos entre los cationes metálicos, el OH superficial y átomos de oxígeno unidos de manera igual por enlaces iónicos y covalentes [20].
La adsorción de los aniones metálicos puede ocurrir en diferentes sitios de las partículas de arcillas, y el lugar puede variar según cada metal [20]. En [20] emplearon modelos de adsorción y deserción para cobre y cadmio en montmorillonitas y encontraron que estos metales eran adsorbidos en los bordes y entre las capas, los autores explican que para cada ión metálico el sitio de preferencia de adsorción puede depender, en diferentes maneras y alcances, de factores como fuerza iónica, el pH y los aniones presentes en la solución.
Modificaciones químicas y físicas de arcillas y zeolitas
La naturaleza química y la estructura de porosa de las arcillas y zeolitas influyen en la capacidad de adsorción; sin embargo, con el fin de aumentar esta capacidad, se realizan modificaciones físicas y químicas. La modificación utilizando agentes químicos normalmente se realiza por un método de impregnación simple [5]. En las investigaciones revisadas se han usado ácidos inorgánicos como el ácido clorhídrico (HCl) o el ácido sulfúrico (H2SO4), como base el dióxido de manganeso (MnO2) y, en el caso de sales, la sal de amoniaco (NH4Cl2) y el cloruro de magnesio (MgCl2).
El objetivo principal de la activación con ácido es obtener un material parcialmente disuelto con una mayor área superficial específica, porosidad y acidez superficial [20]. El lavado con ácido produce la desagregación de las partículas de arcilla, eliminación de impurezas y la disolución de las capas externas [5]; el producto final de este proceso es un silicato hidratado amorfo y poroso con una estructura tridimensional reticulada [20].
En los artículos revisados se ha comprobado que la modificación de la arcilla aumenta la efectividad de remoción de metales pesados, lo que se explica principalmente por un aumento en el área superficial y el volumen de poros. En [21], luego de tratar bentonita con HCl, reportaron un incremento del 57% de superficie específica y una reducción del diámetro promedio del poro en la bentonita ácida; por otro lado, en [2] modificaron la bentonita natural con H2S04, obtuvieron aumentos en la superficie específica y volumen de poros de 3.3 y 2.75 veces respectivamente. El tratamiento con ácido promovió la formación de poros pequeños, lo que incrementó la superficie específica. En [22] trataron caolín con HCl, el análisis realizado comprobó el aumento en número y tamaño de los poros, los valores de área superficial se incrementaron de 15.2 m2/g a 21.4 m2/g para el caolín sin tratamiento y tratado respectivamente. En [6] trataron zeolita con HCl y NaOH, obtuvieron una mayor eficiencia de remoción de plomo con el primer tratamiento, el análisis de superficie específica presentó valores de 68.84, 94.52 y 71.62 m2/g para la zeolita natural tratada con base y ácido respectivamente; por otro lado, la capacidad de intercambio catatónico de la zeolita natural, tratada con NaOH y HCl se midió en 89, 136 y 148 meq/100 g respectivamente. El tratamiento con ácido de las zeolitas remueve las impurezas que bloquean los poros, eliminando progresivamente cationes y removiendo átomos de aluminio conforme la intensidad y duración del tratamiento incrementa [23].
En comparación con el tratamiento con ácido, la impregnación de arcillas con sales y bases es menos usada [5]. Sari y Tüzen [24] estudiaron la adsorción de cadmio con caolín natural y caolín modificado con óxido de manganeso, el análisis BET mostró que el área de superficie específica aumentó en 68% en el caolín modificado. Los autores explican que las fotografías de barrido electrónico del caolín modificado muestran una superficie uniforme debido a que las partículas de dióxido de manganeso ocuparon los espacios y vacíos formados en la superficie durante el tratamiento.
En [25] trabajaron con zeolita modificada con óxido de magnesio y obtuvieron valores de remoción para cadmio, cobre y plomo 1.5 veces mayores en comparación con las zeolitas tratadas con sodio y potasio; al igual que en los casos anteriores la superficie específica aumentó, en este caso, de 27.21 a 62.97 m2/g. La capacidad de adsorción de cadmio, cobre y plomo de la zeolita natural estuvo entre 2.3 y 2.8 mg/g y 3.6 y 4.5 mg/g en la zeolita tratada con calcio y magnesio. Las capacidades de adsorción de las zeolitas modificadas con sodio y potasio fueron similares, los valores más altos se obtuvieron con el tratamiento de magnesio y fueron de, 4.0, 4.5, y 4.3 mg/g para cadmio, cobre y plomo respectivamente.
No en todos los casos un tratamiento físico a la arcilla mejora su eficiencia de adsorción. En [26] impregnaron bentonita con cloruro de amonio y luego la calentaron en un horno a 200 _C durante una hora, en este estudio se aplicaron diferentes rangos de temperatura con valores entre 100 y 200 _C, se produjeron protones suficientes para disolver los elementos minerales de la estructura de la arcilla, lo cual dio lugar a una mayor porosidad y una mejor capacidad de adsorción. Con temperaturas mayores a 200 _C, la descomposición cinética del cloruro de amonio se aceleró incrementando la acidez y destruyendo lo sitios de adsorción y, por ende, la capacidad de remoción de la bentonita. En [27] tuvieron resultados similares cuando compararon la adsorción de bentonita natural contra bentonita calcinada; la bentonita natural fue remojada, tamizada, deshidratada, secada y molida, mientras que la bentonita calcinada se preparó mediante una pirolisis a 650 _C durante tres horas. La bentonita natural mostró una mayor capacidad de intercambio catatónico y adsorción que la bentonita calcinada, tanto en solución multimetal como monometal (0.47 y 0.08 mg/g y 1.82 y 0.50 mg/g); al caracterizar ambos adsorbentes la bentonita natural tenía una mayor capacidad de intercambio catatónico y área de superficie específica. Esta reducción en la superficie de la bentonita calcinada se explicaría por un probable colapso de los poros, mientras que la menor capacidad de intercambio catatónico puede deberse a la liberación de óxidos metálicos de la bentonita a elevadas temperaturas [8].
Parámetros de adsorción
El proceso de adsorción de metales pesados está influenciado por varios factores como el pH, temperatura, presencia de otros elementos, concentraciones iniciales de adsorbente y adsorbato [5]. En las siguientes secciones se desarrollarán estos factores.
Concentración inicial de metales
En los estudios revisados la concentración de metales pesados en la solución muestra una relación inversa con la eficiencia de remoción. En [6] estudiaron la capacidad de adsorción de Pb (II) usando zeolitas tratadas a un pH 6, dosis de adsorbente de 2 g/L y una variación de la concentración inicial de Pb (II) de 10 a 150 mg/L; la cantidad de Pb (II) adsorbida aumentó conforme la concentración de Pb (II) se incrementó de 10 a 50 mg/L, sin embargo, la eficiencia de remoción del Pb (II) se redujo conforme la concentración de este metal varió de 50 a 150 mg/L. Por otro lado, en [2] observaron un comportamiento similar al estudiar la adsorción de Cu (II) y Pb (II) en bentonita. En [25] analizaron la adsorción de Cd (II), Cu (II) y Pb (II) en zeolita modificada con magnesio, con una dosis de 40 mg/L de adsorbente a una concentración de 5 mg/L, tuvieron eficiencias del 100% de remoción para los tres metales; sin embargo, al aumentar la concentración a 10 mg/L y 50 mg/L las eficiencias de remoción estuvieron entre 91-98% y 8-15% respectivamente.
Los resultados anteriores se explican debido a que a bajas concentraciones de adsorbato hay una mayor cantidad relativa de sitios activos disponibles en la superficie del adsorbente para un menor número de iones de adsorbato, a medida que la concentración de sorbato aumenta para una misma dosis de adsorbente, el número relativo de sitios activos se reduce, lo que ocasiona una disminución en el porcentaje de remoción de iones metálicos en solución [28].
pH de la solución
El pH de la solución es uno de los parámetros más importantes en la remoción de metales dado que afecta la carga superficial del adsorbato y adsorbente [29], además de la especiación de los metales en solución [2]. Los iones metálicos tienden a formar complejos con compuestos inorgánicos, el tipo de complejo que se forme dependerá principalmente del valor del pH [30]. Generalmente, pH elevados favorecen la adsorción mediante la formación de cationes hidroxilados. Un pH alto también puede originar que las arcillas presenten una mayor especificidad por metales alcalinos debido a la tendencia de los iones metálicos a hidrolizarse [20].
Cuando la solución se encuentra a un pH bajo el número de iones hidrógeno, supera en gran cantidad a los iones metálicos, la superficie del adsorbente se cubre de iones hidrógeno y se reduce el número de sitios activos disponibles, esto explica la poca adsorción [31]. Más aún, en la bentonita los grupos silanol y aluminol (grupos hidroxilos enlazados con las capas tetraédricas de silicio y octaédricas de aluminio respectivamente) de la superficie tienen más protones y, por tanto, menor capacidad para retener iones metálicos [32]. La zeolita, por su parte, presenta gran cantidad de grupos silanol [33], lo que también contribuye en la cantidad de protones en la superficie. Otro factor en la poca adsorción de iones metálicos a pH bajos, aunque de menor influencia, es el aumento de la densidad de la carga positiva en la superficie del adsorbente por el enlace de los grupos silanol con el hidrógeno, lo que genera repulsión con los iones metálicos cargados positivamente [34].
Conforme aumenta el pH de la solución se reduce la concentración de iones hidrógeno, lo que permite una mejor atracción entre los cationes metálicos (con carga positiva) y la superficie del adsorbente (con carga negativa) [35], donde estos son adsorbidos en la superficie del adsorbente mediante intercambio catatónico o atracción electrostática [36], [37]; sin embargo, a pH muy altos la adsorción de los metales podría estar enmascarada por la precipitación de estos.
En ensayos de adsorción de Cu (II) y Pb (II) en bentonita natural y activada [1], [2], [32], [35] se ha observado que con una variación de pH entre 2 y 4 la adsorción incrementó bruscamente. En la mayor parte de casos estudiados más allá de un pH 6 no se observaron incrementos en la adsorción del Pb (II) [6], [22]; en el caso del Cd (II) se han encontrado valores máximos de adsorción usando bentonita natural con pH cercanos a 6 [1], [35]. En [24] evaluaron también la adsorción Cd (II) en caolín activado, hallando valores máximos de adsorción con pH entre 5 y 7, similares resultados obtuvieron en [38] con zeolita natural.
Las arcillas y zeolitas adsorben iones metálicos mediante dos mecanismos principales, a pH bajos el proceso que domina es el intercambio catatónico entre las capas como resultado de las interacciones entre iones y la carga negativa constante, mientras que a pH altos la remoción de metales ocurre junto con la liberación de iones hidrógeno y, al parecer, es mucho más específica que la adsorción a pH bajos por la formación de complejos en las partículas de arcilla [32]. Sin embargo, en un medio muy alcalino el adsorbente es más susceptible a una hidrólisis, lo que puede inhibir la adsorción. A un pH bajo (entre 3 y 5) el grupo silanol se carga negativamente, lo que facilita la adsorción de cationes metálicos [25]. En suma, el mecanismo de adsorción dependerá en gran parte del pH del medio y tipo de adsorbato y adsorbente, por esta razón es importante hallar el rango de pH óptimo según sea el caso.
Concentración del adsorbente
La tendencia en los estudios revisados es que a mayor cantidad de adsorbente aumenta el porcentaje de metal removido, lo anterior debido al incremento en la cantidad de sitios activos del adsorbente; sin embargo, la capacidad de adsorción por cantidad de adsorbente (mg/g) se reducirá al aumentar la dosis de arcilla o zeolita [2]. Cuando hay una mayor cantidad de concentración de adsorbente, la concentración inicial de metales no alcanza para cubrir todos los sitios de intercambio disponibles en el adsorbente, lo que reduce la capacidad de adsorción [24]; además, una mayor cantidad de adsorbente aumenta la probabilidad de colisión entre las partículas, lo que puede ocasionar una agregación de partículas que reduzca el área superficial y contribuya a una menor capacidad de adsorción de los metales pesados en el adsorbente [39]. La interferencia entre los sitios de intercambio por una mayor concentración de adsorbente también puede reducir la capacidad de adsorción [40].
Otros autores han explicado este comportamiento debido al aumento del pH originado por una mayor cantidad de adsorbente. En [22] usaron como adsorbente caolín activado con ácido (HCl) una concentración inicial de plomo de 100 mg/L y un rango de adsorbente de 0.05 - 0.5 g/L, la disminución de la capacidad de adsorción al aumentar la dosis de caolín se atribuyó a la naturaleza ácida de la superficie del caolín que al adsorber el Pb (II) reduce el pH inicial (al intercambiar iones hidrógenos por cationes de plomo). Con una dosis de 0.05 g/L el pH disminuyó de 4.78 a 4.54 luego de 1440 minutos; con dosis más altas de 0.2 g/L el pH disminuyó aún más, hasta 4.27. Al aumentar la dosis de adsorbente se disminuye aún más el pH lo que hace que los iones metálicos penetren con mayor facilidad en los sitios de adsorción ácidos, reduciendo la remoción de metal por parte del adsorbente [41]. Shaban y Abukhadra [38], usando zeolita natural para la adsorción de Cd (II) a una concentración inicial de 100 mg/L, explicaron igualmente que una mayor cantidad de zeolita en la solución reduce el pH, lo que ocasionará la adición de protones en la superficie de la zeolita disminuyendo la capacidad de adsorción de cationes metálicos.
Temperatura y termodinámica
La temperatura tiene relación directa con la energía cinética de los iones metálicos en solución. Un aumento en la temperatura eleva la tasa de difusión del sorbato, las variaciones de temperatura afectarán el equilibrio de la capacidad de adsorción del adsorbente por un sorbato en particular [42]; generalmente a temperaturas elevadas la remoción de metales aumenta debido a una mayor afinidad del mineral por el metal o un aumento en los sitios activos del adsorbente [43].
El aumento en la temperatura ocasiona cambios en el equilibrio y la cinética, esto se atribuye a tres razones: (a) el aumento en la energía cinética que facilita que los iones metálicos accedan a los sitios activos de adsorción, (b) el aumento en la actividad superficial del adsorbente que promueve e incrementa la afinidad en los sitios activos de adsorción, (c) la disminución de la resistencia de transferencia de masa [12]. Con el aumento de la temperatura se reduce en el grosor de la capa que rodea al adsorbente, lo que al mismo tiempo reduce la resistencia de la transferencia de masa del adsorbato en esta capa, facilitando la difusión del metal en el adsorbente. Conforme la temperatura aumenta, las interacciones electrostáticas se debilitan y los iones reducen su tamaño debido a una menor solvatación [44]; sin embargo, temperaturas muy elevadas pueden dar lugar a daños físicos en el adsorbente, y reducir su capacidad de adsorción [45]. En algunos de los estudios revisados (Tabla II) la temperatura menor presentó la mayor capacidad de adsorción [24], [34], [46], [47].
La termodinámica de adsorción relaciona el equilibrio de adsorción con aquellas propiedades que no pueden medirse directamente en un experimento: la energía de activación, el cambio en la energía de Gibbs estándar 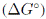 , el cambio en la entalpía estándar
, el cambio en la entalpía estándar 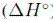 y el cambio en la entropía estándar
y el cambio en la entropía estándar 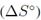 . Uno de los criterios más importantes en la adsorción es el cambio en la energía Gibbs estándar que indica la espontaneidad del proceso. Esta variable se relaciona con la constante de equilibrio de adsorción usando la siguiente ecuación [5]:
. Uno de los criterios más importantes en la adsorción es el cambio en la energía Gibbs estándar que indica la espontaneidad del proceso. Esta variable se relaciona con la constante de equilibrio de adsorción usando la siguiente ecuación [5]:

Donde KD es el coeficiente de distribución de adsorción lineal, definido como la razón entre la concentración en equilibrio del soluto adsorbido en la superficie y la concentración en equilibrio del soluto en la fase líquida. El valor de KD se obtiene graficando ln(qe/CE) contra CE. qe, es la cantidad de soluto adsorbido en la superficie del adsorbente en condición de equilibrio y CE un parámetro de concentración en equilibrio del soluto en solución. El cambio de la entropía estándar se obtiene de la siguiente ecuación [5]:
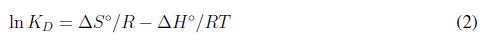
Donde R es la constante de gas, 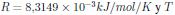 la temperatura absoluta.
la temperatura absoluta.
En la Tabla IIse resumen los valores de los parámetros termodinámicos de adsorción de diversos estudios que usaron como adsorbente bentonita, caolín y zeolita, y como adsorbatos cadmio, cobre y plomo. El valor negativo de 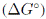 indica la viabilidad y espontaneidad del proceso, mientras que un valor negativo de energía de Gibbs indica lo contrario. De la tabla IIse puede observar que la mayoría de los sistemas presentan valores negativos, indicando que la adsorción de cadmio, cobre y plomo en bentonita, caolín y zeolita es factible y espontánea por naturaleza.
indica la viabilidad y espontaneidad del proceso, mientras que un valor negativo de energía de Gibbs indica lo contrario. De la tabla IIse puede observar que la mayoría de los sistemas presentan valores negativos, indicando que la adsorción de cadmio, cobre y plomo en bentonita, caolín y zeolita es factible y espontánea por naturaleza.
(a) El valor de _G o está dado para la temperatura de adsorción máxima
Tabla II: Parámetros termodinámicos de adsorción de cadmio, cobre y plomo usando bentonita, caolín y zeolita.
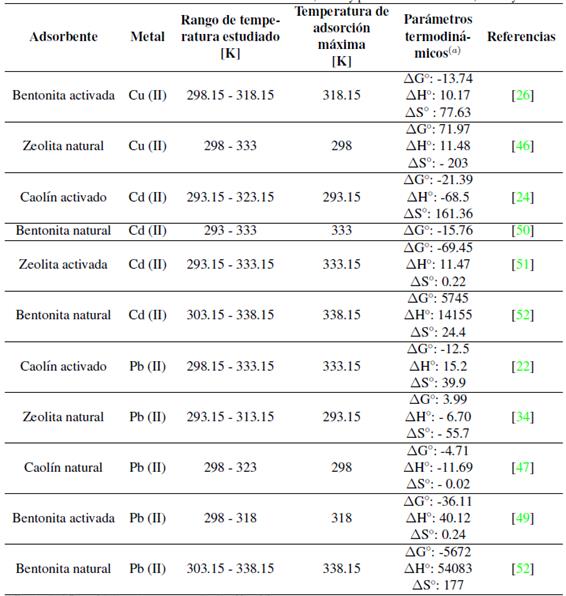
El valor positivo de 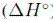 es un indicador de que el proceso de adsorción es endotérmico y de un fuerte enlace entre el soluto y el adsorbente. Un valor negativo de
es un indicador de que el proceso de adsorción es endotérmico y de un fuerte enlace entre el soluto y el adsorbente. Un valor negativo de 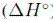 indica que la adsorción es exotérmica, y el enlace entre el soluto y el adsorbente se da principalmente por fuerzas físicas o de Van der Waals. El valor de
indica que la adsorción es exotérmica, y el enlace entre el soluto y el adsorbente se da principalmente por fuerzas físicas o de Van der Waals. El valor de 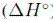 o puede dar idea del tipo de adsorción, entre 5-40 kJ/mol es una adsorción física y entre 40-800 kJ/mol una adsorción química [48]. En la mayoría de casos revisados (Tabla II) el valor positivo indica un proceso endotérmico y valores que revelan una predominancia de la adsorción física.
o puede dar idea del tipo de adsorción, entre 5-40 kJ/mol es una adsorción física y entre 40-800 kJ/mol una adsorción química [48]. En la mayoría de casos revisados (Tabla II) el valor positivo indica un proceso endotérmico y valores que revelan una predominancia de la adsorción física.
El valor de 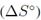 indica la aleatoriedad del sistema. Un valor positivo de
indica la aleatoriedad del sistema. Un valor positivo de 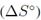 o refleja una alta afinidad del soluto en los sitios de adsorción, así como un aumento en la aleatoriedad de la interface sólido-solución durante la adsorción; este es el caso de la mayoría de estudios revisados (Tabla II). Generalmente el valor de
o refleja una alta afinidad del soluto en los sitios de adsorción, así como un aumento en la aleatoriedad de la interface sólido-solución durante la adsorción; este es el caso de la mayoría de estudios revisados (Tabla II). Generalmente el valor de 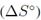 o en el sistema aumenta luego de un proceso de modificación. Un valor negativo de
o en el sistema aumenta luego de un proceso de modificación. Un valor negativo de 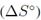 o indica una menor aleatoriedad en el sistema de adsorción [5]; antes de que ocurra la adsorción, los iones metálicos cercanos a la superficie del adsorbente estarán más ordenados que en un estado adsorbido. La relación entre los iones metálicos libres interactuando con el adsorbente será mayor que en un estado adsorbido, como resultado, la distribución de la energía rotacional y traslacional entre un pequeño número de moléculas aumentará conforme aumente la adsorción, lo que aumentará la aleatoriedad en la interface sólido-solución durante el proceso de adsorción [49].
o indica una menor aleatoriedad en el sistema de adsorción [5]; antes de que ocurra la adsorción, los iones metálicos cercanos a la superficie del adsorbente estarán más ordenados que en un estado adsorbido. La relación entre los iones metálicos libres interactuando con el adsorbente será mayor que en un estado adsorbido, como resultado, la distribución de la energía rotacional y traslacional entre un pequeño número de moléculas aumentará conforme aumente la adsorción, lo que aumentará la aleatoriedad en la interface sólido-solución durante el proceso de adsorción [49].
Adsorción competitiva
En sistemas multicomponentes la capacidad de adsorción usualmente será menor que en un sistema de un componente, es decir, los cationes metálicos tienen un efecto antagonista en la adsorción. En [53] estudiaron la adsorción competitiva usando 1 g/L de bentonita, una concentración de 5 a 250 mg/L de Ni (II) y Cu (II) y de 5 a 150 mg/L de Pb (II), todo a un pH 5 y tiempo de contacto de 120 minutos. La interacción entre los metales se evaluó relacionando la capacidad de adsorción del metal en estudio con y sin la existencia de otros iones metálicos. Para analizar el efecto sinérgico o antagonista de los metales dividieron la capacidad máxima de adsorción en solución multimetal y la capacidad máxima de adsorción en solución monometal 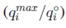 cuando este valor fuera mayor a uno el efecto sería sinérgico, igual a uno sin efecto y menor a uno un efecto antagonista. Los valores de esta razón para los cuatro metales estudiados fueron menor a uno (entre 0.229 y 0.290) lo que demuestra el efecto antagonista en los cuatro sistemas estudiados, es decir, los iones metálicos compiten por los sitios de adsorción de la bentonita entre ellos, y la adsorción se inhibe en presencia de otros iones. Se calculó también el porcentaje de reducción de adsorción en la solución multimetal respecto a la solución monometal. Las reducciones fueron de 75.39, 72.02, 71.01 y 77.06% para Cd (II), Cu (II), Ni (II) y Pb (II) respectivamente. Al relacionar
cuando este valor fuera mayor a uno el efecto sería sinérgico, igual a uno sin efecto y menor a uno un efecto antagonista. Los valores de esta razón para los cuatro metales estudiados fueron menor a uno (entre 0.229 y 0.290) lo que demuestra el efecto antagonista en los cuatro sistemas estudiados, es decir, los iones metálicos compiten por los sitios de adsorción de la bentonita entre ellos, y la adsorción se inhibe en presencia de otros iones. Se calculó también el porcentaje de reducción de adsorción en la solución multimetal respecto a la solución monometal. Las reducciones fueron de 75.39, 72.02, 71.01 y 77.06% para Cd (II), Cu (II), Ni (II) y Pb (II) respectivamente. Al relacionar 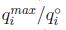 con la concentración inicial de metales se observa una relación inversa, a medida que aumenta la concentración de metales la razón disminuye, es decir, a mayor presencia de metales en solución el efecto antagonista es cada vez mayor, incluso para el Pb (II) a concentraciones muy bajas (5 mg/L) el efecto era sinérgico (una razón positiva). Los autores explican que este comportamiento se debe a que a bajas concentraciones los metales son adsorbidos en sitios específicos, conforme aumenta la concentración de metales, la superficie del adsorbente pierde su habilidad de atrapar metales pesados por el traslape de sitios de adsorción, reduciendo la especificidad por un metal en particular. Esto, en suma, reduce la adsorción.
con la concentración inicial de metales se observa una relación inversa, a medida que aumenta la concentración de metales la razón disminuye, es decir, a mayor presencia de metales en solución el efecto antagonista es cada vez mayor, incluso para el Pb (II) a concentraciones muy bajas (5 mg/L) el efecto era sinérgico (una razón positiva). Los autores explican que este comportamiento se debe a que a bajas concentraciones los metales son adsorbidos en sitios específicos, conforme aumenta la concentración de metales, la superficie del adsorbente pierde su habilidad de atrapar metales pesados por el traslape de sitios de adsorción, reduciendo la especificidad por un metal en particular. Esto, en suma, reduce la adsorción.
En [27] estudiaron la adsorción de Pb (II), Cd (II) y Mn (II) en bentonita natural. En el ensayo emplearon 50 mg de arcilla seca junto con 50 mL de solución metálica a diferentes concentraciones, la mezcla se agitó a 100 rpm por 72 h; igualmente en solución multimetal la capacidad de adsorción fue menor que en solución monometal. La adsorción de Pb (II) en solución multimetal disminuyó de 0.046 a 0.036 mmol/g. La acumulación total de metales en la superficie de la bentonita en solución multimetal fue de 9.85 mg/g, lo que representa un aumento de 5.38% respecto a la adsorción de Pb (II) en sistema monometal (9.32 mg/g). Lo anterior significa que la adsorción de Pb (II), en comparación con el Cd (II) y el Mn (II), está muy poco afectada por la presencia de cationes competitivos, estos resultados le permiten afirmar a los autores que la adsorción de Pb (II) en bentonita es selectiva en cuanto al sitio de adsorción. El hecho de que en la solución multimetal no se observe la dominancia de un metal en específico por los sitios activos, señalan los autores, indica que el adsorbente presenta sitios de adsorción específicos para cada catión metálico. Los autores concluyen que a pesar de la competencia que existe con otros cationes, cada metal es capaz de colocarse en su sitio de adsorción específico, aun a pesar del aumento en la concentración de metales.
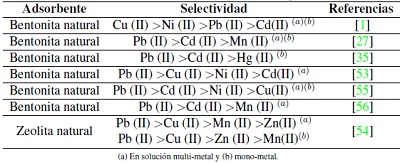
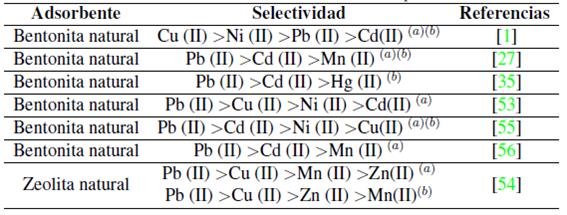
Otro estudio sobre adsorción competitiva lo realizaron Zendelska y Golomeova [54] utilizando zeolita natural y una solución multimetal de Cu (II), Mn (II), Pb (II) y Zn (II) a una concentración inicial de 25 mg/L, un pH inicial de 3.5 y una dosis de adsorbente de 5 g en 400 mL, la solución se centrifugó a 400 rpm durante 360 minutos. La cantidad de adsorción en el caso del Cu (II) se redujo en 10% en la solución multimetal respecto a la solución monometal; en el caso del Zn (II) y el Mn (II), las disminuciones fueron de 25 a 50 %; para el Pb (II), la diferencia de adsorción entre la solución multimetal y monometal fue mínima, asimismo, la cantidad total de metales adsorbidos por unidad de masa en la solución multimetal fue mayor que en la solución monometal. Según los autores la diferencia en la capacidad de adsorción de los iones metálicos puede deberse a factores como el radio de hidratación, la entalpía de hidratación y la solubilidad de los cationes. El radio de hidratación de los cationes es: 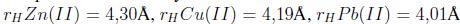
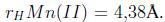 Idealmente los cationes más pequeños deberían adsorberse más rápido y en mayor cantidad que los cationes más grandes, debido a que los cationes más pequeños pueden pasar con mayor facilidad a través de los microporos y capas de la estructura de la zeolita. Los autores indican que la adsorción debería describirse según la entalpia de hidratación; que es la energía que permite que las moléculas de agua se desprendan de los cationes y, por ende, refleja la facilidad con que los cationes interactúan con el adsorbente. Debido a la gran cantidad de silicios respecto al aluminio en la zeolita, esta tiene una densidad de carga estructural baja, por esta razón, los cationes metálicos con una hidratación de energía baja van a ser adsorbidos en mayor cantidad que aquellos de energía alta. La energía de hidratación de los cationes es: -2010, -1955, -1760 y -1481 kJ/mol para Cu(II), Zn(II), Mn(II) y Pb(II) respectivamente. Según los valores de energía de hidratación y radio de hidratación la zeolita preferirá al Pb antes que al Cu, Mn o Zn en una solución multimetal; por esta razón se espera que concentraciones elevadas de Pb limiten la remoción de Cu, Mn y Zn. Este análisis hecho por Zendelska y Golomeova [54] concuerda con los estudios revisados (Tabla III) donde el plomo usualmente es el primero en orden de selectividad ya sea en soluciones multi o mono metal.
Idealmente los cationes más pequeños deberían adsorberse más rápido y en mayor cantidad que los cationes más grandes, debido a que los cationes más pequeños pueden pasar con mayor facilidad a través de los microporos y capas de la estructura de la zeolita. Los autores indican que la adsorción debería describirse según la entalpia de hidratación; que es la energía que permite que las moléculas de agua se desprendan de los cationes y, por ende, refleja la facilidad con que los cationes interactúan con el adsorbente. Debido a la gran cantidad de silicios respecto al aluminio en la zeolita, esta tiene una densidad de carga estructural baja, por esta razón, los cationes metálicos con una hidratación de energía baja van a ser adsorbidos en mayor cantidad que aquellos de energía alta. La energía de hidratación de los cationes es: -2010, -1955, -1760 y -1481 kJ/mol para Cu(II), Zn(II), Mn(II) y Pb(II) respectivamente. Según los valores de energía de hidratación y radio de hidratación la zeolita preferirá al Pb antes que al Cu, Mn o Zn en una solución multimetal; por esta razón se espera que concentraciones elevadas de Pb limiten la remoción de Cu, Mn y Zn. Este análisis hecho por Zendelska y Golomeova [54] concuerda con los estudios revisados (Tabla III) donde el plomo usualmente es el primero en orden de selectividad ya sea en soluciones multi o mono metal.
(a) En solución multi-metal y (b) mono-metal.
Tabla III: Selectividad de iones metálicos con diferentes tipos de adsorbente
Eficiencias de remoción
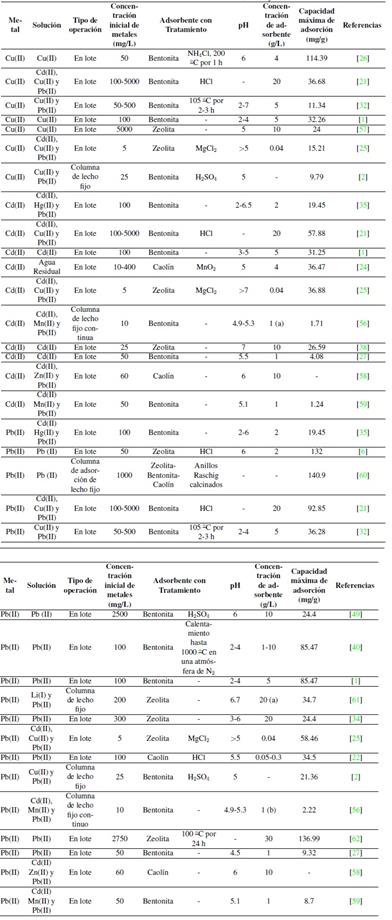
Como se ha visto, la capacidad de adsorción depende de los parámetros de operación. En la Tabla IVse resumen las eficiencias de remoción de cadmio, cobre y plomo empleando bentonita, caolín y zeolita en diferentes condiciones.
(a) El valor representa gramos a un flujo de 6.7 mL/min y (b) a un flujo de 1.4 mL/min.
Tabla IV: Eficiencias de remoción de bentonita, caolín y zeolita.
Isotermas
La isoterma de adsorción es la ecuación o curva que, en condición de equilibrio, relaciona la concentración de metal adsorbida en la fase sólida con la concentración de metal en solución para una temperatura específica [12], lo anterior se conoce como el equilibrio de adsorción y es uno de los aspectos más importantes a analizar en la adsorción [5], pues permite predecir la eficiencia del proceso y optimizar el uso del adsorbente [12]. La cantidad de iones metálicos adsorbidos en el adsorbente dependerá de la concentración en equilibrio de los iones metálicos, pH de la solución en equilibrio, y temperatura [63]. Una descripción matemática precisa para describir la isoterma de adsorción permitirá diseñar sistemas de adsorción eficientes [64]. Los modelos de equilibrio pueden clasificarse en modelos empíricos y mecánicos; así, los modelos empíricos, a pesar de no poder representar los mecanismos de adsorción, se pueden usar para predecir resultados experimentales, estos modelos se basan en relaciones matemáticas simples entre la fase líquida en concentración de equilibrio y la fase sólida en concentración de equilibrio [12]. En la Tabla Vse resumen los modelos de dos y tres parámetros usados para describir la adsorción de plomo, cadmio y cobre en bentonita, caolín y zeolita en los estudios revisados. Los supuestos de estos modelos han sido desarrollados ampliamente por otros autores [6], [65].
qe
: concentración en equilibrio de metal en la fase sólida (mg/g)
qmax
: capacidad máxima de adsorción (mg/g)
Ce
: concentración en equilibrio de metal en la fase líquida (mg/L) R: constante de gas universal (J/molK) T: temperatura absoluta (K)
Cs
: solubilidad del soluto para una temperatura determinada
Eo
: energía promedio de adsorción por mol de adsorbato (kJ/mol)
Tabla V: Modelos de equilibrio de adsorción.
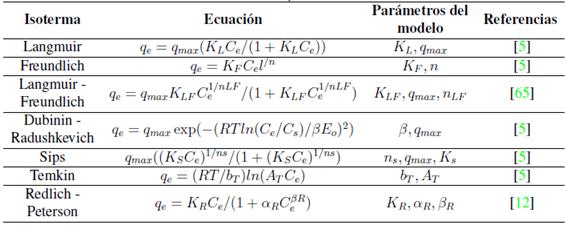
Para la mayoría de casos las isotermas de Langmuir y Freundlich son las que mejor se ajustan a los datos, este alto nivel de correlación podría significar que durante el proceso de adsorción se forman monocapas y multicapas en la superficie del adsorbente. La mayoría de estudios han usado modelos de dos parámetros dada la complejidad de usar los modelos de tres parámetros. La práctica común es analizar la adsorción según los modelos de Langmuir y Freundlich [1], [2], [22], [25], [32], [47], [49], [60], [62], incluso algunos solo usan el modelo de Langmuir [6], [21]. Algunos autores han empleado también, además de los dos modelos comúnmente usados, el de Dubinin-Radushkevich (D-R) [24], [26], [40], [57], [66], este modelo permite hallar la variación de energía del proceso, para esos estudios los valores estuvieron entre 8-16 kJ/mol, lo que indica la naturaleza química del proceso. En [35] analizaron la adsorción de metales pesados con los modelos de Langmuir, Freundlich, Temkin y D-R; las isotermas de mejor ajuste variaron según el metal. Shaban y Abukhadra [38] usaron los modelos de Langmuir, Freundlich y Temkin; finalmente, en [27] analizaron los modelos Langmuir, Freundlich, Redlich-Peterson y Temkin.
Es importante señalar que en la mayoría de estudios el equilibrio de adsorción se analiza con las formas lineales de los modelos de isotermas. Es probable que al usar las formar no lineales de estos modelos se obtengan resultados diferentes de capacidad máxima de adsorción [12]. Actualmente, no existen estudios comparativos que apliquen ambos modelos, lineales y no lineales, de isotermas de dos parámetros para la adsorción de cadmio, cobre y plomo en arcillas y zeolitas.
Cinética
Uno de los factores más importantes para el diseño de un sistema de adsorción es predecir la tasa a la cual ocurrirá la adsorción, el tiempo de residencia del adsorbato y las dimensiones del reactor dependerán de la cinética del sistema de adsorción [12]. La cinética de adsorción se expresa como la velocidad de remoción de soluto que controla el tiempo de residencia del soluto en la interfase sólido-solución [5]. En los estudios de adsorción es importante identificar los mecanismos involucrados que pueden incluir difusión externa, difusión interna y reacciones químicas [12], para ellos existen modelos cinéticos que se basan en la superficie de reacción como el paso cinético que controla la tasa de adsorción. Estos modelos incluyen el cinético de pseudoprimer orden (también llamado el modelo Lagergren), el cinético de pseudosegundo orden, la ecuación de Elovich y de difusión intraparticular (Tabla VI).
qt
: concentración de metal en la fase sólida a un tiempo t (mg/g) t: tiempo en minutos C: intercepto
Tabla VI: Modelos de cinética de adsorción.
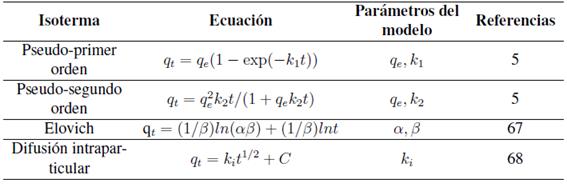
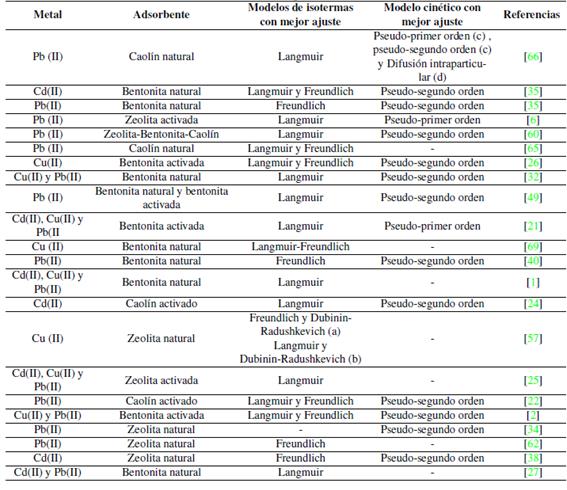
Como se aprecia en la Tabla VIIlas ecuaciones de pseudoprimer y pseudosegundo orden son las más usadas para correlacionar los datos de adsorción de cadmio, cobre y plomo en arcillas y zeolitas. En la mayoría de los casos el modelo de pseudo-segundo orden es el que mejor describe la adsorción, solo unos pocos estudios [6], [21], [64] reportan que el modelo de pseudoprimer orden se ajusta mejor a los datos.
(a) Cuando la concentración inicial de Cu (II) estuvo entre 500 y 1000 mg/L y (b) mayor a 1000 mg/L
Tabla VII: Modelos de equilibrio de adsorción.
Predecir la fase limitante de la velocidad de adsorción es un factor importante a ser considerado en el proceso. En la adsorción, la transferencia del soluto se caracteriza usualmente ya sea por una transferencia masa externa (difusión en la capa límite) o una difusión intraparticular o ambas [70]. Puede asumirse que en los mecanismos de remoción de metales pesados por adsorción ocurren los siguientes pasos [71]: (a) migración del adsorbato del soluto a la superficie del adsorbente, (b) difusión del adsorbato a través de la capa límite a la superficie del adsorbente, (c) adsorción del adsorbato en un sitio activo de la superficie del adsorbente, (d) difusión intraparticular del adsorbato hacia el interior de la estructura porosa del adsorbente. La fase más lenta será la que controle el proceso. La cuarta fase ocurre muy rápido y no influye en la velocidad de difusión, la primera fase es también muy rápida dado que los iones metálicos están disponibles en la capa límite; entonces, la velocidad de adsorción estará definida ya sea por la segunda o tercer fase, o también por ambas [72], de aquí la relevancia de aplicar el modelo de difusión intraparticular.
Al finalizar su estudio en [40] concluyeron que el modelo de difusión intraparticular no era un factor determinante en la cinética de adsorción del plomo en bentonita natural. Similares resultados obtuvieron [22] al estudiar la adsorción de plomo en caolín activado.
En [64] usaron caolín como adsorbente de Pb (II), para la mayor concentración inicial ensayada (80, 160 y 320 mg/L) el modelo de difusión intraparticular se ajustó mejor a los datos. Esto demuestra que, tal y como se postula en la ley de Fick, a mayor concentración habrá una mayor difusión. En [46], con el fin de estudiar la adsorción de Cu (II) en zeolita natural, emplearon tres modelos de difusión: intraparticular, homogénea y parabólica. Los autores concluyen que durante los 60 primeros minutos la curva cinética se ajustó al modelo de difusión intraparticular y al de difusión homogénea, esto ocurre, explican los autores, debido a que inicialmente (de 0 a 60 minutos) la difusión en la capa superficial de la partícula sucede de manera simultánea con la difusión a través de la capa límite; luego de los primeros 60 minutos ocurre la difusión a través de los poros del adsorbente hacia los sitios de intercambio. Por último, dado que el modelo de difusión parabólica mostró una linealidad en todos los ensayos, este se usó para calcular los parámetros termodinámicos. En [52] usaron el modelo de difusión intraparticular para determinar si era un factor determinante en la cinética de adsorción del cadmio, manganeso y plomo en bentonita natural. Concluyeron que ese era el caso, sin embargo, se indicó que la capa límite podría representar también cierta restricción a la difusión de metales.
Conclusiones
El uso de bentonita, caolín y zeolita es efectivo para la remoción de cadmio, cobre y plomo de soluciones acuosas. Las modificaciones químicas y físicas que se realizan en las arcillas y zeolitas aumentan la capacidad de adsorción y superficie específica de los materiales; sin embargo, se han reportado algunos casos en que la eficiencia de adsorción se reduce luego de un tratamiento del mineral. Se han elaborado numerosos estudios para evaluar el impacto de diversos parámetros de adsorción, el análisis realizado ha mostrado que los parámetros de operación más importantes son la concentración inicial de metales, el pH de la solución, la dosis de adsorbente y temperatura del sistema. La presencia de otros iones metálicos en la solución usualmente impacta de manera negativa la adsorción, sin embargo, en el caso del plomo no parece verse afectado por la presencia de otros iones metálicos. La influencia de diversos parámetros de operación se ve reflejado en la variabilidad de las capacidades de adsorción de cadmio, cobre y plomo como se resume en las tablas presentadas.
En relación con el equilibrio de adsorción en la mayoría de estudios revisados, la isoterma de Langmuir es la que mejor se ajusta a los datos y, en menor cantidad de trabajos, la isoterma de Freundlich. Algunos investigadores han usado otras isotermas como los modelos de D-R, Sips y Temkin para predecir el equilibrio de adsorción. Muchos estudios han modelado la cinética de adsorción con las ecuaciones de pseudoprimer orden y pseudosegundo orden, en la mayor parte de estudios el modelo de pseudosegundo orden es el que mejor se ajusta a los datos. El modelo de difusión intraparticular también ha sido usado para estudiar la adsorción.
La tendencia en estudios de adsorción se concentra en la elaboración de nanoadsorbentes, dado que estos presentan una mayor área superficial de adsorción y, por ende, un mayor rendimiento; sin embargo, una de las principales debilidades de estos compuestos es la dificultad en recuperarlos y la toxicidad que podrían representar en el agua tratada, por esta razón se considera pertinente el estudio de la adsorción de compuestos naturales haciendo énfasis en su uso en escalas piloto y real para promover así el empleo extendido en plantas de tratamiento de aguas residuales.
Referencias
License
From the edition of the V23N3 of year 2018 forward, the Creative Commons License "Attribution-Non-Commercial - No Derivative Works " is changed to the following:
Attribution - Non-Commercial - Share the same: this license allows others to distribute, remix, retouch, and create from your work in a non-commercial way, as long as they give you credit and license their new creations under the same conditions.




2.jpg)