DOI:
https://doi.org/10.14483/udistrital.jour.colomb.for.2014.1.a08Publicado:
01-01-2014Número:
Vol. 17 Núm. 1 (2014): Enero-JunioSección:
Artículos de investigación científica y tecnológicaChemical modification of Calophyllum brasiliense Cambess. and Enterolobium cyclocarpum (Jacq.) Griseb. wood
Modificación química de madera de Calophyllum brasiliense Cambess. y Enterolobium cyclocarpum (Jacq.) Griseb
Palabras clave:
acetilación, anhídrido acético, madera tropical, modificación química (en).Descargas
Referencias
Azeh, Y., Olatunji, G., Mohammed, C., & Mamza, P. A. (2013). Acetylation of wood flour from four wood species grown in Nigeria using vinegar and acetic anhydride. International Journal of Carbohydrate Chemistry, 1-6.
Blanco, M., Carpio, I., & Muñoz, F. (2005). Fichas técnicas de veinte especies maderables de importancia comercial en Costa Rica. San José: Universidad de Costa Rica. 106 p.
Evans, P., Wallis, F. A., & Owen, N. L. (2000). Weathering of chemically modified wood surfaces. Natural weathering of Scots pine acetylated to different weight gains. Wood Science. Technology., 34, 151–165.
Garay, R., & Henriquez, M. (2012). Tratamiento químico de acetilación en madera de Pinus Radiata. Maderas, Ciencia y Tecnología, 1 (14), 102-113.
García, L., Guindeo, A., & Peraza, C. (2003). La madera y su anatomía. Madrid: Editorial Mundi-Prensa / Fundación Salazar. 320 p
Hill, C. (2006). Wood modification. Chemical, thermal and other processes. West Sussex: John Wiley and Sons. 240 p
Hill, C., Cetin, N., & Ozmen, N. (2000). Potential catalysts for the acetylation of wood. Holzforschung, 54, 269-272.
Hill, C., Forster, S., Farahani, M., Hale, M., Ormondroyd, G., & Williams, G. (2005). An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. International. Biodeterioration. Biodegradation, 55, 69-76.
Hon, D. N. S. (1996). Chemical modification of lignocellulosic materials. New York: Marcel Dekker Inc., 370 p
Instituto de Pesquisas Tecnológicas (IPT) (2001). Biodeterioração de madeiras em edificacões. São Paulo: IPT, 54 p
Jebrane, M., Harper, D., Nicole, L., & Sébe, G. (2011). Comparative determination of the grafting distribution and viscoelastic properties of wood blocks acetylated by vinyl acetate or acetic anhydride. Carbohydrate. Polymers., 84, 1315-1320.
Jebrane, M., & Sébe, G. (2007). A novel simple route to wood acetylation by transesterification with vinyl acetate. Holzforschung, 61 (2), 143-147.
Ohkoshi, M. (2002). FTIR-PAS study of light-induced changes in the surface of acetylated or polyethylene glyco-impregnated wood. Journal. Wood Science., 48, 394-401.
Pardo, T. (2013). Evaluación del efecto de la concentración de anhídrido acético, temperatura y tiempo en la reacción de acetilación para la modificación química de Tectona grandis y Gmelina arborea (Licentiate thesis for Chemical Engineering). San José: Universidad de Costa Rica. 190 p
Papadopoulos, A. N., & Pougioula, G. (2010). Mechanical behaviour of pine wood chemically modified with a homologous series of linear chain carboxylic acid anhydrides. Bioresource Technology, 101, 6147-6150.
Peterson, M. D., & Thomas, R. J. (1978). Protection of wood from decay fungi by acetylation-an ultrastructural and chemical study. Wood Fiber Science, 10 (3), 149-163.
Mitsui, K. (2010). Acetylation of wood causes photobleaching. Journal. Photochemistry. Photobiology. B, 101, 210-214.
Stefke, B., Windeisen, E., Schwanninger, M., & Hinterstoisser, B. (2008). Determination of the weight percentage gain and of the Acetyl group content of Acetylated wood by means of different infrared spectroscopic methods. Analytical. Chemistry., 80 (4), 1272-1279.
Rowell, R. M. (2005). Chemical modification of wood. In R. M. Rowell (ed.). Handbook of wood chemistry and wood composites. Boca Raton, FL: CRC Press, 487 p.
Rowell, R. M. (2006). Acetilation of wood. Forest Products. Journal., 56 (9), 4-12.
Rowell, R. M., Youngquist, J. A., & Imamura, Y. (1988). Strength tests on acetylated aspen flakeboards exposed to a brown-rot fungus.Wood Fiber Science, 20 (2), 266-271.
Technical Association of Pulp and Paper Industry (TAPPI) (1992a). Acid-insoluble lignin in wood and pulp. Standard method: T-222 om-88. Atlanta, GA: TAPPI Press.
Technical Association of Pulp and Paper Industry (TAPPI) (1992b). Ash in wood and pulp. Standard method: T-211 om-93. Atlanta, GA: TAPPI Press.
Technical Association of Pulp and Paper Industry (TAPPI) (1992c). Preparation of wood for chemical analysis. Standard method: T-264 om-88. Atlanta, GA: TAPPI Press.
Yurkanis, P. (2006). Organic chemistry (5th ed.). New York: Prentice Hall, 1270 p.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
 |
Blanco, E., Alfaro, J. (2014). Chemical modification of Calophyllum brasiliense Cambess. and Enterolobium cyclocarpum (Jacq.) Griseb. wood. Colombia Forestal, 17(1), 125-132. |
CHEMICAL MODIFICATION OF Calophyllum brasiliense Cambess. AND Enterolobium cyclocarpum (Jacq.) Griseb. WOOD
Modificación química de madera de Calophyllum brasiliense Cambess. y Enterolobium cyclocarpum (Jacq.) Griseb
Short title: Chem. mod. Caloph. bras. and Enter. cycl.
Ernesto Blanco1, Johnny Alfaro2
1Escuela de Ingeniería Química, Universidad de Costa Rica, Ciudad Universitaria Rodrigo Facio, San Pedro, Costa Rica, P.O.Box: 2060 Costa Rica.
2Laboratorio de Productos Forestales, Universidad de Costa Rica, Ciudad Universitaria Rodrigo Facio, San Pedro, Costa Rica, P.O.Box: 2060
Costa Rica. Institute for Molecular Engineering, University of Chicago, Chicago, IL, USA, 60637. alfaro@uchicago.edu, to whom correspondence
should be addressed.
Recepción: 1 de noviembre de 2013 / Aprobación: 30 de diciembre de 2013
ABSTRACT
Wood samples of two tropical hardwood species, Cedar Mary (Calophyllum brasiliense Cambess.) and Guanacaste (Enterolobium cyclocarpum (Jacq.) Griseb.), growing in Costa Rica were chemically modified by acetylation. Acetic anhydride was used as reagent and donor of the acetyl group, potassium carbonate as a catalyst and N,N-dimethylformamide (DMF) as a solvent. The reaction was carried out at 90° C and three reaction times were studied in order to determine the percentage weight gain (PWG) for every treatment. For the seven hour reaction, PWG was 12.88 % and 12.44 % for Guanacaste and Cedar Mary samples respectively. IR spectra using ATR technology were obtained in order to corroborate the reaction. Results showed characteristic signals of functional groups C = O (1737 cm-1), C-O (1220 cm-1) and C-H bending vibration for the acetyl group (1368 cm-1), which indicates that hydroxyl groups had been replaced by acetyl groups. Longer reaction times increase this replacement in both species.
Keywords: acetylation, acetic anhydride, Tropical hardwood, chemical modification.
RESUMEN
Se modificaron muestras de madera de las especies latifoliadas Cedro María (Calophyllum brasiliense Cambess.) y Guanacaste (Enterolobium cyclocarpum (Jacq.) Griseb.), que crecen en Costa Rica, por medio de la reacción de acetilación. Se utilizó anhídrido acético como reactivo y donador del grupo acetil, carbonato de potasio como catalizador y N,N-dimetilformamida (DMF) como solvente. La reacción se llevó a cabo a 90 °C y se estudiaron tres tiempos de reacción, con la finalidad de determinar el porcentaje de ganancia en peso (WPG, por sus siglas en inglés) para cada tratamiento. Para siete horas de reacción el WPG obtenido fue de 12.88 y 12.44 % para las muestras de Guanacaste y Cedro María, respectivamente. Se obtuvieron espectros IR utilizando tecnología ATR para confirmar la modificación química. Los resultados permiten observar cambios en las señales características de los grupos funcionales C = O (1737 cm-1), C-O (1220 cm-1) y C-H para la vibración del grupo acetil (1368 cm-1), los cuales brindan evidencia de que los grupos hidroxilos fueron reemplazados por los grupos acetil, así como que tiempos de reacción prolongados conllevan un aumento del intercambio de los grupos funcionales para ambas especies.
Palabras clave: acetilación, anhídrido acético, madera tropical, modificación química.
INTRODUCTION
The wood has many technical advantages for a great variety of applications because it has a high specific resistance, good hardness, high rigidity, low processing energy, the capacity for renovation and aesthetic beauty (Stefke et al., 2008). At the same time, it has many different natural enemies that include fungi, termites, marine borers, humidity, UV radiation, and fire. Nevertheless, treatments against these plagues have been developed in order to in-crease the lifetime of wood products (IPT, 2001).
Traditional solutions against these problems involve the use of chemical preservatives. This has been discouraged because of growing environmental concerns, particularly since non-toxic compounds can change the structure of wood, and toxicity related problems can be avoided through the chemical modification of wood (Peterson & Thomas, 1978).
The chemical modification of wood is a treatment that consists of a reaction between a reagent and the polymeric structural components of wood, thus resulting in the formation of a covalent bond between a reagent and wood. In the case of acetylation, the product has acetyl groups instead of hydroxyl groups (OH) in the cell walls of wood (Rowell, 2006).
Wood acetylation has proved to be an effective method that enhances the performance of wood (Hon, 1996; Hill, 2006). Furthermore, wood acetylation using acetic anhydride (AA) as reagent has received considerable attention, and commercial acetylated wood products have been produced in Europe since 2007 (Jebrane et al., 2011).
Recent studies have demonstrated that dimensional stability, resistance to fungal attack and photo stability of wood can be enhanced using this treatment (Evans et al., 2000; Hill et al., 2005; Ohkoshi, 2002). Most recently vinyl acetate has also been used as reagent, since its by-product is not an acid (Jebrane & Sébe, 2007).
The most studied acetylation reaction consists in the use of a small quantity of AA in liquid phase, with or without a catalyst. The reaction has been studied in flakes, sawdust (Azeh et al., 2013; Jebrane et al., 2011) and solid samples of different sizes (Hill et al., 2000; Mitsui, 2010; Garay & Henriquez, 2012).
The economic benefit of acetylation when compared to other reactions is that AA is used in small quantities, which implies a lesser use of reagents and energy for heating purposes (Rowell, 2005).
The reaction occurs on site and only in one direction, which implies that the hydroxyl groups present in the wood structure are replaced by the acetyl groups without polymerization. Due to the non polymerization nature of the reaction, all weight gain in the wood is directly related to the hydroxyl groups substituted (Rowell, 2005).
While the reaction takes place, an expansion in the cell wall occurs, therefore allowing other active sites of the structure to be available for chemical modification. In this reaction, for each molecule of AA that reacts, one molecule of acetic acid is produced. Since acetic acid has the capacity to act as a swelling agent, an additional mechanism is available for this expansion. The use of a catalyst increases the reaction rate and thus the production of acetic acid, which in many cases introduces a secondary reaction between AA and the acetic acid (Rowell, 2005).
Previous research studies in acetylation have centered on the use of softwoods (Rowell et al.,1988; Papadopoulos & Pougioula, 2010; Jebrane et al., 2011), which are not native of countries with tropical weather, for example, in Costa Rica. Thus, the main purpose of the present work is to treat wood of the tropical hardwood species Cedar Mary (Calophyllum brasiliense Cambess.) and Guanacaste (Enterolobium cyclocarpum (Jacq.) Griseb), the former being a species that can be found in Central America, Colombia, Venezuela and Brazil and the latter a native species of Costa Rica, using AA with three reaction times in order to calculate its WPG and to obtain IR spectra which can corroborate the hydroxyl group substitution for both species.
MATERIAL AND METHODS
MATERIALS AND CHEMICALS
Samples were prepared of C. brasiliense wood obtained from the Atlantic region in Costa Rica and E. cyclocarpum wood obtained from North Pacific region in Costa Rica. Sawdust from both species was prepared for chemical characterization using standard method TAPPI: T-264 om-88. Acid insoluble lignin content was determined using standard method TAPPI: T-222 om-88 and ash content was determined using standard method TAPPI: T-211 om-93. Holocellulose (cellulose plus hemicellulose) was determined by difference.
Wood samples were prepared with dimensions 15.5 mm x 15.5 mm x 10.5 mm, which were treated with an extraction with 250 mL of a mixture of benzene/ ethanol/acetone (4:1:1 v/v) for 8 hours and two extractions with 250 mL of distilled water for 8 and 7 hours, respectively. Then the wood samples were oven dried at 103°C for 16 hours and cooled to room temperature in a desiccator containing silica.
ACETYLATION REACTION
The acetic anhydride (AA) concentration for each gram of dry wood was calculated based on the amount of hydroxyl groups to be substituted, which can be calculated according to Pardo (2013). For each gram of dry wood, the reaction mixture consisted of 1.75 mL AA, 7 mL N,N-dimethylformamide (DMF) and 0.0207 g K2CO3. DMF was dried with molecular sieves of 4 Å in order to se-cure an anhydrous environment. The samples were then added and subjected to vacuum/atmospheric pressure cycles in order to pre-impregnate the wood with the reagent solution.
The reaction’s temperature was fixed at 90°C and the values of the time of reaction were fixed as two, four and a half, and seven hours with the purpose of determining the influence of this parameter over WPG. Thirty two samples were prepared for each reaction time.
The reactions were performed in a round-bottomed flask equipped with a condenser and a drying tube with calcium chloride. Once the reaction was finished, samples were extracted by means of a Soxhlet equipment using a benzene/ethanol/acetone (4:1:1 v/v) solution for 8 hours and then with distilled water for 7 hours in order to remove unreacted compounds, DMF, and by-products as much as possible.
The blocks were oven dried at 103°C for 16 hours and cool to room temperature, thus the weight percent gain (WPG) was calculated as:
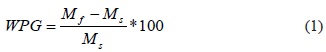
where Ms = oven dried weight of the untreated block
Mf = oven dried weight of the acetylated wood.
Acetic anhydride, DMF, toluene and benzene were purchased to Costa Rica’s representative of J.T. Baker and ethanol was purchased to Gamma Re-actives in San Jose, Costa Rica. The dimensions of the sample were measured before and after the treatment and the initial and final volume was calculated, thus the volume percent gain (VPG) was defined as:

where V1 = initial volume before the treatment.
V2 = final volume of sample after the treatment.
IR SPECTROSCOPY
Spectra were obtained using an IR spectrophotometer with ATR technology in the range of 4000 and 650 cm-1, with a resolution of 0.5 cm-1. Two samples were selected randomly for each reaction time and two untreated samples were prepared, thus having eight samples for each species in order to study the behavior of OH and acetyl groups as the reaction progresses.
RESULTS
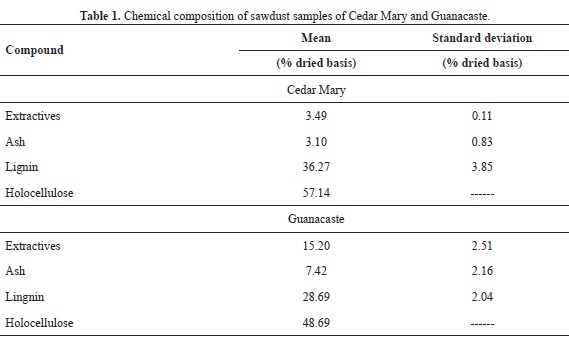
The results of the chemical characterization for both Cedar Mary and Guanacaste are showed in Table 1. The data from Table 1 was used in order to determine the amount of theoretical hydroxyl groups that are available for the reaction with AA using the calculations proposed in Pardo (2013), and a 30% excess was used in order to ensure that the reaction took place, yielding 1.75 mL per gram of dry wood.
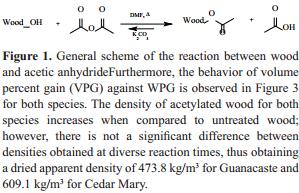
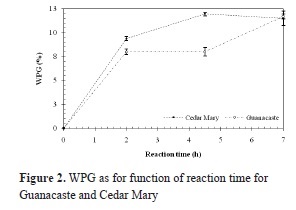
Once the amount of AA was calculated, wood from both tropical species reacted with AA according to the scheme in Figure 1. Figure 2 shows the relation between weight percent gain (WPG) and reaction time for Guanacaste and Cedar Mary wood.
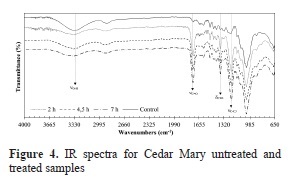
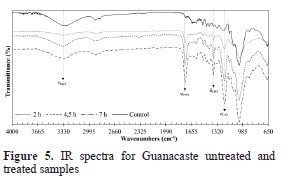
On the other hand, IR spectra were obtained using ATR technology in order to offer an evidence of the progressive substitution of hydroxyl groups over the reaction time and with the purpose of determining the progressive appearance of acetyl groups in treated blocks. The spectra for Cedar Mary samples are shown in Figure 4 and for Guanacaste samples in Figure 5.
DISCUSSION
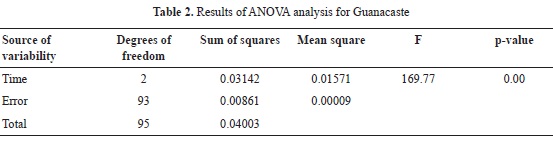
The data for Guanacaste shown in Table 1 are very close to those reported in Blanco et al (2005), and the data for both species do not deviate from values that are normal for hardwood species (García et al., 2003).
Although Guanacaste wood is more porous, from Figure 2 it can be observed that WPGs are lower for similar reaction times than for Cedar Mary wood, thus having a lower reaction rate.
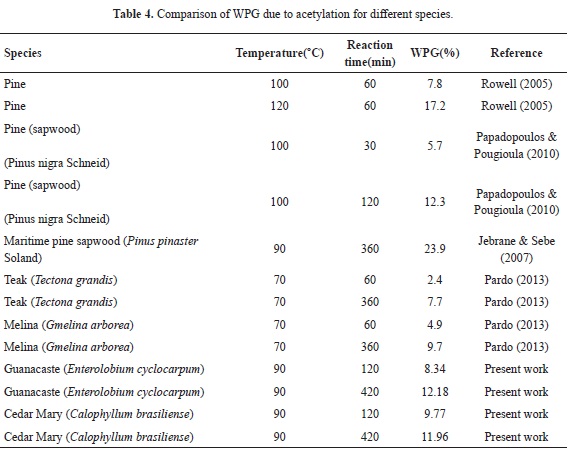
Mean WPG for 2, 4,5 and 7 hours of reaction for Guanacaste wood are 8.34, 8.35 and 12.18% respectively; in the case of Cedar Mary wood, mean WPG are 9.77, 12.44 and 11.96%. In both cases, values are relatively low, when compared to WPG of softwood species reported in the literature, as presented in Table 2. When compared to other hardwood species (Teak and Melina), Cedar Mary and Guanacaste have higher WPG as shown in Table 2.
From Figure 2 it can also be inferred that WPG increases with reaction time and that the mean weight gain is achieved during the first two hours of acetylation for both species, which is similar to the three hours observed for other tropical hardwood species Teak and Melina, reported by Pardo (2013); however, longer reaction times will be required before it can be concluded that the reaction has reached chemical equilibrium.
Moreover, when WPG of both species is compared to the WPG of softwood species reported in the literature, both hardwoods have lower values, as presented in Table 4; e.g., for maritime pine sapwood treated at the same temperature and 6 hours, WPG is double compared to the WPG obtained for both species at 7 hours
Furthermore, when reaction times are compared between softwood species (Rowell, 2005; Papadopoulos & Pougioula, 2010) and Cedar Mary and Guanacaste, it can be found that WPG are smaller for these hardwood species with lower temperatures of reaction. These lower WPG are surprisingly unexpected since hardwoods have a morphological structure which should help diffusion of AA and thus increase WPG for an equal reaction time. Besides, when compared to other hardwood species, for example, Teak and Melina that Pardo (2013) used, WPG are relatively higher because these species are more porous.
Since acetyl groups have a larger molar volume compared to the hydroxyl groups that are substituted, the wood’s cell wall suffers an expansion according to longer reaction times which is related to higher WPG; thus, an increase in dimensions is observed.
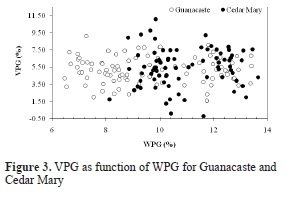
As shown in Figure 3, VPG due to acetylation does not have a particular trend with WPG for both species; however, VPG in Guanacaste increases with reaction time until a maximum mean value of 6.23% is obtained for 7 hours of reaction. On the other hand, VPG for Cedar Mary increases with reaction time as well, but a maximum mean value of 6.84% is reached at 4.5 hours of reaction and beyond that time a decrease in WPG is observed.
Furthermore the effectiveness of this treatment against natural enemies as termites or white-rot fungus (which affect more commonly hardwoods) is related to WPG and it should be studied for both species.
For both species, the untreated (control) samples in Figures 4 and 5 exhibit a strong signal at wavenumbers between 3333 cm-1 and 3336 cm-1, which is characteristic of stretching vibrations of OH present in lignin, cellulose and hemicellulose. Also the asymmetric stretch band that is characteristic of sp3 carbons in methyl group (Yurkanis, 2006) is present.
In the fingerprint region, a signal at 1733 cm-1 is present due to stretching vibrations of the acetate group. The next band is due to C=C bond present in aromatic rings of lignin between 1593 cm-1 and 1506 cm-1. Other bands observed are at 1421 cm-1 caused by bending of CH2 in cellulose and hemicellulose, at 1317 cm-1 due to deformation of CH3, stretching of bond C-O-C of esters at 1157 cm-1 and C-O vibration of primary alcohols of cellulose and hemicellulose at 1030 cm-1.
For both species, the treated samples in Figures 4 and 5 have a strong signal at 1737 cm-1 that is due to C=O bonds that are introduced during the acetylation reaction. This band becomes more intense (lower values of transmittance) as reaction time increases. Further, a decrease in the band at 3335 cm-1 due to OH groups is observed as reaction time increases. Another signal that appears is due to the acetyl group at 1220 cm1, which belongs to the C-O bond. This signal also increases with longer reaction time.
Therefore, the results clearly evidence that the reaction has occurred as it was supposed to occur, and that at higher reaction times the interchange between hydroxyl and acetyl group increases.
CONCLUSIONS
In conclusion, the reactivity of both species is different, although the reaction was conducted with the same conditions of temperature and quantity of reagent. Likewise, WPG for 7 hours of reaction time was 11.24% for Cedar Mary and 12.18% for Guanacaste. It is also important to make experiments with longer reaction times in order to determine if these will be required before it can be concluded that the reaction has reached chemical equilibrium. Although Guanacaste is more porous than Cedar Mary, it presents a slower reaction rate. In addition, IR spectra have provided enough evidence to conclude that the reaction is occurring as expected and that interchange between OH and acetyl groups increases with reaction time. Also, the behavior of these acetylated hardwoods against natural enemies such as termites and white-rot fungi should be studied.
AKNOWLEDGMENTS
This research was funded by Vicerrectoría de Investigación de la Universidad de Costa Rica from the research project number 731-B2-242. The authors also thank the Centro de Electroquímica y Energía Química de la Universidad de Costa Rica -CELEQfor the help provided in facilitating the equipment to obtain IR spectra.
BIBLIOGRAPHICAL REFERENCES
Azeh, Y., Olatunji, G., Mohammed, C., & Mamza, P. A. (2013). Acetylation of wood flour from four wood species grown in Nigeria using vinegar and acetic anhydride. International Journal of Carbohydrate Chemistry, 1-6.
Blanco, M., Carpio, I., & Muñoz, F. (2005). Fichas técnicas de veinte especies maderables de importancia comercial en Costa Rica. San José: Universidad de Costa Rica. 106 p.
Evans, P., Wallis, F. A., & Owen, N. L. (2000). Weathering of chemically modified wood sur-faces. Natural weathering of Scots pine acetylated to different weight gains. Wood Science Technology, 34, 151-165.
Garay, R., & Henríquez, M. (2012). Tratamiento químico de acetilación en madera de Pinus Radiata. Maderas, Ciencia y Tecnología, 1 (14), 102-113.
García, L., Guindeo, A., & Peraza, C. (2003). La madera y su anatomía. Madrid: Editorial Mundi-Prensa / Fundación Salazar. 320 p.
Hill, C. (2006). Wood modification. Chemical, thermal and other processes. West Sussex: John Wiley and Sons. 240 p.
Hill, C., Cetin, N., & Ozmen, N. (2000). Potential catalysts for the acetylation of wood. Holzforschung, 54, 269-272.
Hill, C., Forster, S., Farahani, M., Hale, M., Ormondroyd, G., & Williams, G. (2005). An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. International Biodeterioration Biodegradation, 55, 69-76.
Hon, D.N.S. (1996). Chemical modification of lignocellulosic materials. New York: Marcel Dekker Inc. 370 p.
Instituto de Pesquisas Tecnológicas -IPT-(2001). Biodeterioração de madeiras em edificacões. São Paulo: IPT, 54 p.
Jebrane, M., Harper, D., Nicole, L., & Sébe, G. (2011). Comparative determination of the grafting distribution and viscoelastic properties of wood blocks acetylated by vinyl acetate or acetic anhydride. Carbohydrate Polymers, 84, 1315-1320.
Jebrane, M., & Sébe, G. (2007). A novel simple route to wood acetylation by transesterification with vinyl acetate. Holzforschung, 61 (2), 143-147.
Ohkoshi, M. (2002). FTIR-PAS study of lightinduced changes in the surface of acetylated or polyethylene glyco-impregnated wood. Journal Wood Science, 48, 394-401.
Pardo, T. (2013). Evaluación del efecto de la concentración de anhídrido acético, temperatura y tiempo en la reacción de acetilación para la modificación química de Tectona grandis y Gmelina arborea (Licentiate thesis for Chemical Engineering). San José: Universidad de Costa Rica. 190 p.
Papadopoulos, A. N., & Pougioula, G. (2010). Mechanical behaviour of pine wood chemically modified with a homologous series of linear chain carboxylic acid anhydrides. Bioresource Technology, 101, 6147-6150.
Peterson, M. D., & Thomas, R. J. (1978). Protection of wood from decay fungi by acetylationan ultrastructural and chemical study. Wood Fiber Science, 10 (3), 149-163.
Mitsui, K. (2010). Acetylation of wood causes photobleaching. Journal Photochemistry Photobiology. B, 101, 210-214.
Stefke, B., Windeisen, E., Schwanninger, M., & Hinterstoisser, B. (2008). Determination of the weight percentage gain and of the Acetyl group content of Acetylated wood by means of different infrared spectroscopic methods. Analytical Chemistry, 80 (4), 1272-1279.
Rowell, R.M. (2005). Chemical modification of wood. In R. M. Rowell (ed.). Handbook of wood chemistry and wood composites. Boca Raton, FL: CRC Press, 487 p.
Rowell, R.M. (2006). Acetilation of wood. Forest Products Journal., 56 (9), 4-12.
Rowell, R.M., Youngquist, J. A., & Imamura, Y. (1988). Strength tests on acetylated aspen flakeboards exposed to a brown-rot fungus. Wood Fiber Science, 20 (2), 266-271.
Technical Association of Pulp and Paper Industry -TAPPI-(1992a). Acid-insoluble lignin in wood and pulp. Standard method: T-222 om 88. Atlanta, GA: TAPPI Press.
Technical Association of Pulp and Paper Industry -TAPPI-(1992b). Ash in wood and pulp. Standard method: T-211 om-93. Atlanta, GA: TAPPI Press.
Technical Association of Pulp and Paper Industry -TAPPI-(1992c). Preparation of wood for chemical analysis. Standard method: T-264 om-88. Atlanta, GA: TAPPI Press.
Yurkanis, P. (2006). Organic chemistry (5th ed.). New York: Prentice Hall, 1270 p.
Licencia
Colombia Forestal conserva los derechos patrimoniales (copyright) de las obras publicadas, y favorece y permite la reutilización de las mismas bajo la licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional por lo cual se pueden copiar, usar, difundir, transmitir y exponer públicamente, siempre que:
Se reconozcan los créditos de la obra de la manera especificada por el autor o el licenciante (pero no de una manera que sugiera que tiene su apoyo o que apoyan el uso que hace de su obra).