DOI:
https://doi.org/10.14483/2256201X.18064Publicado:
01-01-2022Número:
Vol. 25 Núm. 1 (2022): Enero-junioSección:
Artículos de investigación científica y tecnológicaCambios en calidad del suelo asociados a la aplicación de técnicas de agricultura ecológica en plantas café bajo diferentes coberturas
Changes in Soil Quality Associated with the Implementation of Ecological Agriculture Techniques in Coffee Plants under Different Coverings
Palabras clave:
agroecología, enzimas, sistemas agroforestales, microorganismos, revolución verde (es).Palabras clave:
agroecology, enzymes, agroforestry systems, microorganisms, green revolution (en).Descargas
Referencias
Alcaldía Municipal San José de Pare Boyacá (2020). Municipio San José de Pare. http://www.sanjosedepare-boyaca.gov.co/municipio/nuestro-municipio
Alejo-Iturvide, F., Márquez-Lucio, M. A., Gonzáles-López, C. I., & de la Rivadela, G. A. (2016). Biosolubilizadores de fósforo orgánico e inorgánico del bosque templado de Santa Rosa Guanajuato, México. Revista de Ciencias Ambientales y Recursos Naturales, 2(3), 10-17. https://www.ecorfan.org/spain/researchjournals/Ciencias_Ambientales_y_Recursos_Naturales/vol2num3/Revista_de_Ciencias_Ambientales_y_Recursos_Naturales_V2_N3_2.pdf
Avellaneda-Torres, L. A., Guevara-Pulido, C. P., & Torres-Rojas, E. (2014). Assessment of cellulolytic microoganisms in soils of nevados Park, Colombia. Brazilian Journal of Microbiology, 45(4), 1211-1220. https://doi.org/10.1590/S1517-83822014000400011 DOI: https://doi.org/10.1590/S1517-83822014000400011
Avellaneda-Torres, L. A., León-Sicard, T. E., & Torres-Rojas, E. (2018). Impact of potato cultivation and cattle farming on physicochemical parameters and enzymatic activities of neotropical high Andean Páramo ecosystems soils. Science of the Total Environment, 631-632, 1600-1610. https://doi.org/10.1016/j.scitotenv.2018.03.137 DOI: https://doi.org/10.1016/j.scitotenv.2018.03.137
Avellaneda-Torres, L., León-Sicard, T., Guerra-Castro, E., & Torres-Rojas, E. (2020). Potato cultivation and livestock effects on microorganism functional groups in soils from the neotropical high Andean Páramo. Revista Brasileira de Ciência di Solo, 44, 1-25. https://doi.org/10.36783/18069657rbcs20190122 DOI: https://doi.org/10.36783/18069657rbcs20190122
Ávila-Bayona, C. P., Álvarez-Cano, M. A., & Avellaneda-Torres, L. M. (2020). Soil Quality Indicators Associated With The Application Of Mycorrhizal Fungi In Coffee Plantations. Revista Ingeniería Solidaria, 44(3), 1-26 : https://doi.org/10.16925/2357-6014.2020.03.05 DOI: https://doi.org/10.16925/2357-6014.2020.03.05
Bautista, A., Etchevers, J., del Castillo, R., & Gutiérrez, C. (2004). La calidad del suelo y sus indicadores. Revista Científica y Técnica de Ecología y Medio ambiente, 13(2), 90-97. http://www.revistaecosistemas.net/articulo.asp?Id=149
Bernal, F. & Forero, U. (2014). Evaluación de especies vegetales para el manejo de la acidez en suelos sulfatados ácidos de Paipa, Boyacá. Corpoica ciencias tecnológicas agropecuarias, 15(2), 229-236. https://doi.org/10.21930/rcta.vol15_num2_art:362 DOI: https://doi.org/10.21930/rcta.vol15_num2_art:362
Caballero-Vanegas, J. J., Mejía-Zambrano, K. B., & Avellaneda-Torres, L. M. (2018). Effect of ecological and conventional managements on soil enzymatic activities in coffee agroecosystems. Pesquisa Agropecuária Tropical, 48(4), 420-428. https://doi.org/10.1590/1983-40632018v4852373 DOI: https://doi.org/10.1590/1983-40632018v4852373
Callisaya, Y. & Fernández, C. (2017). Evaluación del efecto que tienen los microorganismos eficientes (EM), en el cultivo de pepinillo (Cucumis sativus L.), municipio de Achocalla. APTHAPI, 3(3), 652-666. http://ojs.agro.umsa.bo/index.php/ATP/article/view/161
Casanova-Lugo, F., Petit-Aldana, J., & Solorio-Sánchez, J. (2011). Los sistemas agroforestales como alternativa a la captura de carbono en el trópico mexicano. Revista Chapingo serie ciencias forestales y del ambiente, 17(1), 133-143. https://doi.org/10.5154/r.rchscfa.2010.08.047 DOI: https://doi.org/10.5154/r.rchscfa.2010.08.047
CONABIO (2015). Bosque, selvas y cafés de Chiapas. Comisión Nacional para el Conocimiento y Uso de la biodiversidad, México. https://www.biodiversidad.gob.mx/corredor/cbmm/pdf/bosques_selvas_cafes_chiapas.pdf
Ceccon, E. (2008). La revolución verde: tragedia en dos actos. Ciencias, 1(91), 21-29. https://www.redalyc.org/pdf/644/64411463004.pdf
de los Ríos, I., Becerril, H., & Rivera, M. (2016). La agricultura ecológica y su influencia en la prosperidad rural: visión desde una sociedad agraria (Murcia, España). Agrociencia, 50(3), 375-389. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952016000300375&lng=es&tlng=es.
Doran, J. & Parkin, T. (1994). Biochemical fractionation of soil organic metter after incorporation of organic residues. Open Journal of Soil Science, 5(6), 135-143.
Federación Nacional de Cafeteros de Colombia (FNC) (2012). Comportamiento de la industria Cafetera Colombiana 2012. https://federaciondecafeteros.org/static/files/Informe_Industrial_Completo2012.pdf
Fonseca-López, D., Bohórquez-Masmela, I. A., Rodríguez-Molano, C. E., & Vivas-Quilla, N. J. (2020). Efecto del periodo de recuperación en la producción y calidad nutricional de algunas especies forrajeras. Biotecnología en el sector agropecuario y agroindustrial, 18(2), 135-145. https://doi.org/10.18684/BSAA(18)135-144 DOI: https://doi.org/10.18684/BSAA(18)135-144
García, J. & Ballesteros, M. (2006). Evaluación de los parametros de calidad para la determinación de fosforo disponible en suelos. Revista Colombiana de Química, 35(1), 81-89. https://www.redalyc.org/articulo.oa?id=309026667006García, Y., Ramírez, W., & Sánchez, S. (2012). Indicadores de la calidad de los suelos: una nueva manera de evaluar este recurso. Pastos y Forrajes, 35(2), 125-138. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-03942012000200001
Garzón, A. F., Perdomo-Rivas, L. P., and Avellaneda-Torres, L. M. (2020). Effect of management (ecological and conventional) on functional groups of soil microorganisms in coffee agroecosystems with different resilience to climate variability, Colombia. Acta Scientiarum. Biological Sciences, 42(1), e48620. https://doi.org/10.4025/actascibiolsci.v42i1.48620 DOI: https://doi.org/10.4025/actascibiolsci.v42i1.48620
Geissert, D., Mólgora, A., Negrete, S., & Hunter, R. (2017). Efecto del manejo de la cobertura vegetal sobre la erosión hídrica en cafetales de sombra. Agrociencia, 51(2), 119-133. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952017000200119&lng=es&tlng=es
González, A., Rivera, M., Ortiz, C., Almaraz, J., Trujillo, A., & Cruz, G. (2013). Uso de fertilizantes orgánicos para la mejora de propiedades químicas y microbiológicas del suelo y del crecimiento del cítrico Citrange troyer. Universidad y ciencia, 29(2), 123-139. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0186-29792013000200003
Guiberteau, A. & Labrador, J. (1991). Técnicas de Cultivo en Agricultura Ecológica. Ministerio de Agricultura Pesca y Alimentación Secretaría General de Estructuras Agrarias.
Gutiérrez, J., Aguilera, L., & González, C. (2008). Agroecología y sustentabilidad. Convergencia, 15(46), 51-87. http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1405-14352008000100004&lng=es&nrm=iso
Gutiérrez, V., Pinzón, A., Cassas, J., & Mercedes, M. (2008). Determinación de la actividad celulolítica del suelo proveniente de cultivos de Stevia rebaudiana Bertoni. Agronomía Colombiana, 26(3), 497-504. https://revistas.unal.edu.co/index.php/agrocol/article/view/12014/12639
Henao, E. (2020). Los sistemas agroforestales expuestos como sistemas sostenibles de producción en Colombia (Trabajo de grado, Ingeniería Agroforestal). Universidad Nacional Abierta y a Distancia UNAD.
IDEAM (1994). Medición de la humedad del suelo. In IDEAM (Eds.). Guía de prácticas hidrológicas (195-206). IDEAM. http://documentacion.ideam.gov.co/openbiblio/bvirtual/012406/Cap15.pdf
Jarquín, A., Salgado, S., Palma, D., Camacho, W., & Guerrero, A. (2011). Análisis de nitrógeno total en suelos tropicales por espectroscopía de infrarrojo cercano (NIRS) y quimiometría. Agrociencia, 45(6), 653-662. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952011000600001
Jaramillo, D. (2011). Caracterización de la materia orgánica del horizonte superficial de un andisol hidromórfico del oriente antioqueño (Colombia). Revista Académica Colombiana de Ciencias, 35(134), 23-33.
Kassambara, A. & Mundt, F. (2020). Factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7. https://CRAN.R-project.org/package=factoextra
Liriano, R., Núñez, D., Hernandez, L., & Castro, A. (2015). Evaluación de microorganismos eficientes y Trichoderma harzianum en la producción de posturas de cebolla (Allium cepa L). Centro agrícola, 42(2), 25-32. http://oaji.net/articles/2016/2674-1454357776.pdf
Martínez, E., Fuentes, J. P., & Acevedo, E. H. (2008). Carbono orgánico y propiedades del suelo. Revista de la ciencia del suelo y nutrición vegetal, 8(1), 68-96. https://doi.org/10.4067/S0718-27912008000100006 DOI: https://doi.org/10.4067/S0718-27912008000100006
Milanés, M., Rodríguez, H., Ramos, R., & Rivera, M. (2005). Efectos del compost vegetal y humus de lombriz en la producción sostenible de capítulos florales en Calendula officinalis L y Matricaria recutita L. Revista Cubana de Plantas Medicinales, 10(1). http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962005000100008
Monserrate, A., Zambrano, D., Rondón, A., Laurencio, M., Pérez, Q., León, R., & Rivera, R. (2014). Aislamiento, selección y caracterización de hongos celulolíticos a partir de muestras de suelo en Manabí-Ecuador. Revista de facultad de ciencias agrarias, 46(2), 177-189. https://www.redalyc.org/pdf/3828/382837658004.pdf
Múnera, G. (2014). El fósforo elemento indispensable para la vida vegetal. Universidad Tecnológica de Pereira. http://repositorio.utp.edu.co/dspace/bitstream/handle/11059/5248/el%20fosforo%20elemento.pdf?sequence=1&isAllowed=y
Narváez, M., Sánchez M., & Menjivar, J. (2010). Cambios en las Propiedades Químicas y en la Actividad de las Fosfatasas en Suelos Cultivados con Maíz Dulce (Zea mays L) Fertilizados con Vinaza. Revista Facultad Nacional de Agronomía Medellín, 63(2), 5533-5541. https://revistas.unal.edu.co/index.php/refame/article/view/25042
Patiño, C. & Sanclemente, O. (2014). Los microorganismos solubilizadores de fósforo (MSF): una alternativa biotecnológica para una agricultura sostenible. Entramado, 10(2), 288-297. https://www.redalyc.org/pdf/2654/265433711018.pdf
Puertas, F., Arévalo, E., Zúñiga, L., Alegre, J., Loli, O., Soplin, H., & Baligar, V. (2008). Establecimiento de cultivos de cobertura y extracción total de nutrientes en un suelo de trópicos húmedo en la Amazonía Peruana. Ecología aplicada, 7(1,2), 23-28. https://doi.org/10.21704/rea.v7i1-2.356 DOI: https://doi.org/10.21704/rea.v7i1-2.356
R Core Team (2019). A language and environment for statistical computing. R Foundation for Statistical Computing.
Rennie, R. (1981). A single medium for the isolation of acetylene-reducing (dinitrogen-fixing) bacteria from soils. Canadian Journal of Microbiology, 27(8), 8-14. https://doi.org/10.1139/m81-002 DOI: https://doi.org/10.1139/m81-002
Restrepo, G., Marulanda, S., de la Fe, Y., Díaz, A., Lucia, V., & Hernández, A. (2015). Bacterias solubilizadoras de fosfato y sus potencialidades de uso en la promoción del crecimiento de cultivos de importancia económica. Revista CENIC Ciencias Biológicas, 46(1), 63-76. https://www.redalyc.org/pdf/1812/181238817006.pdf
Rosatto, L., de Mello, R., Castellanos, L., Reyes, A., Caione, G., & Silva, C. (2014). Solubilización de fuentes de fósforo asociadas a un compuesto orgánico enriquecido con biofertilizantes. Agrociencia, 48(5), 489-500. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952014000500003
Rubio-Sánchez, W. R. (2016). Impactos de la revolución verde en la agricultura colombiana. https://nutriciongeneral.files.wordpress.com/2016/06/revolucic3b3n-verde.pdf
Ruelas-Mojardín, L. C., Nava-Tablada, M. E., Cervantes, J., & Barradas, V. L. (2014). Importancia ambiental de los agroecosistemas cafetaleros bajo sombra en la zona central montañosa del estado de Veracruz, México. Madera y bosques, 20(3), 27-40. https://doi.org/10.21829/myb.2014.203149 DOI: https://doi.org/10.21829/myb.2014.203149
Salinas-Vásquez, F., Sepúlveda-Morales, L., & Sepúlveda-Chavera, G. (2014). Evaluación de la calidad química del humus de lombriz roja californiana (Eiseniafoetida) elaborado a partir de cuatro sustratos orgánicos en Arica. Idesia (Arica), 32(2), 95-99. https://doi.org/10.4067/S0718-34292014000200013 DOI: https://doi.org/10.4067/S0718-34292014000200013
Sundara, R. & Sinha, M. (1963). Phosphate dissolving microorganisms in the soil and rhizosfere. Indian Journal of Agricultural Sciences, 1(33), 272 – 278.
Tapia, Y. & García, F. (2013). La disponibilidad del fósforo es producto de la actividad bacteriana en el suelo en ecosistemas oligotróficos. Una revisión crítica. Terra Latinoamericana, 31(3), 231-242. http://www.scielo.org.mx/scielo.php?pid=S0187-57792013000400231&script=sci_abstract
Trejo-Escareño, H. I., Salazar-Sosa, E., López-Martínez, J. D., & Vázquez-Vázquez C. (2013). Impacto del estiércol bovino en el suelo y producción de forraje de maíz. Revista mexicana de ciencias agrícolas, 4(5), 727-738. https://doi.org/10.29312/remexca.v4i5.1171 DOI: https://doi.org/10.29312/remexca.v4i5.1171
Vela, G. López, J., & Rodríguez, M. (2012). Niveles de carbono orgánico total en el suelo de conservación del Distrito Federal, central de México. Investigaciones Geográficas, Boletín del Instituto de Geografía, UNAM, 77, 18-30. http://www.scielo.org.mx/pdf/igeo/n77/n77a3.pdf DOI: https://doi.org/10.14350/rig.31007
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Recibido: 27 de mayo de 2021; Aceptado: 17 de agosto de 2021
Abstract
The aim of this research was to evaluate the changes in the soil quality associated with the implementation of ecological agriculture (EA) techniques in coffee plantations under different coverings (banana, mandarin, and a mix of citrus fruits) in San José de Pare, Boyacá. To this effect, changes in physicochemical parameters, the abundance of functional groups of microorganisms (phosphate solubilizers, cellulolytic microorganisms, nitrogen fixers), and enzymatic activities (acid and alkaline phosphatase, β-glucosidase, urease, and protease) were determined. The soil samples were collected on a bimonthly basis for a period of ten months in plots cultivated with coffee plantations with three different coverings: banana, mandarin, and a mix of citrus fruits. The results showed modifications in the soil quality indicators as a consequence of the application of EA techniques, finding a significant increase in the content of phosphorus, carbon, phosphate solubilizing bacteria and fungi, and cellulolytic fungi. In contrast, no significant changes were observed for enzymatic activity.
Keywords:
agroecology, enzymes, agroforestry systems, microorganisms, green revolution..Resumen
La presente investigación tuvo como objetivo evaluar cambios de la calidad del suelo asociados a la implementación de técnicas de agricultura ecológica (AE) en cafetales bajo diferentes coberturas en San José de Pare, Boyacá. Para el cumplimiento de este objetivo se determinaron cambios en parámetros fisicoquímicos, abundancia de grupos funcionales de microorganismos (solubilizadores de fosfatos, celulolíticos, fijadores de nitrógeno) y actividades enzimáticas (fosfatasa acida y alcalina, β-glucosidasa, ureasa y proteasa). Las muestras de suelo se recolectaron de manera bimensual durante un periodo de diez meses en parcelas con cultivo de café con tres diferentes coberturas: plátano, mandarina y una mezcla de cítricos. Los resultados mostraron modificaciones de los indicadores de calidad del suelo como consecuencia de la aplicación de técnicas de AE, encontrando un aumento significativo en el contenido de fósforo, carbono, bacterias y hongos solubilizadores de fosfato y hongos celulolíticos. En contraste, no se observaron cambios significativamente relevantes en la actividad enzimática.
Palabras clave:
agroecología, enzimas, sistemas agroforestales, microorganismos, revolución verde.Introduction
Ecological Agriculture (EA) aims for a natural and friendly relationship with the environment to promote plant and animal biodiversity (de los Ríos et al., 2016), unlike the Green Revolution, which is associated with the extensive use of chemical fertilizers and heavy-duty machinery (Ceccon, 2008). This has led agriculture to several negative impacts, creating simplified and unstable ecosystems and causing environmental deterioration (Rubio-Sánchez, 2016).
It is in this context that EA uses techniques which are favorable to the environment with changes in crop rotation and the use of green manures, vermicomposts (worm humus), poultry manure, biofertilizers, and efficient microorganisms (EM) in order to maintain soil productivity (Guiberteau & Labrador, 1991).
Another productive and environmentally friendly strategy is the implementation of agroforestry systems, also called SAF, a sustainable alternative that helps increase soil productivity (Casanova-Lugo et al., 2011). In addition, these systems contribute to multiple environmental services such as mitigating the conflict of land use in Colombia, especially considering that our country has a great diversity of species. This allows for the diversification of said systems, of which coffee production is one of the predominant modalities (Henao, 2020).
These traditional techniques use the resources that are available, namely organic fertilizers, in this case worm humus, which can be an efficient solution to replace mineral fertilizers, as they help maintain balance in the soil (Milanés et al., 2005). Another technique that has been implemented is the use of biofertilizers that improve and conserve the physical, chemical, and biological conditions of the soil, given that a large part of the organic matter comes from enzymatic digestion by microorganisms, and it is from this organic matter that we obtain the nutrients that are easily assimilated by the soil (Trejo-Escareño et al., 2013).
Moreover, EMs are also used as organic fertilizers in agriculture, with the great benefit of increased production, in addition to helping prevent and reduce the attack of pests and diseases. They are produced as a mixture of natural resources that is highly efficient and beneficial (Callisaya and Fernández, 2017). Finally, poultry manure provides benefits such as increased moisture retention, increased organic matter, and improved soil fertility through increased amounts of macro and micronutrients (González et al., 2013).
It has been suggested that the implementation of these EA techniques, as well as the different covering options, provides several benefits to the soil. According to the National Commission for Biodiversity Knowledge and Use (Comisión nacional para el conocimiento y uso de la biodiversidad) (CONABIO, 2015), shade-grown coffee is particularly promising in terms of conservation, with benefits such as greater biomass, more soil nutrients, and microbial biodiversity, fewer weeds and harmful insects, and a better water and microclimate balance. Trees like orange, banana, and guava, among others, generate benefits to the soil by providing organic matter produced by leaf litter and atmospheric nitrogen fixation (Ruelas-Mojardín, 2014). In Colombia, coffee is a product with special altitude, latitude, and climate conditions. This means that Colombian coffee culture has one fundamental requirement: the quality of the soil (FNC, 2012). This is why the agroecological conditions of San José de Pare, Boyacá, are suitable for coffee plantations; it has a cultivated area of 617 ha, with plants cultivated under the shade or coverings due to the high radiation that is emitted in this area (Alcaldía Municipal San José de Pare Boyacá, 2020).
Soil is one of the most important resources for life on the planet (García et al., 2012). Therefore, physicochemical indicators such as gravimetric moisture, pH, CEC, phosphorus, organic carbon, and nitrogen are so important, since they allow determining the degree of vulnerability of a given soil depending on the activities it has been exposed to. This includes the buffering capacity of the soil and the availability of water and nutrients for plants and microorganisms. Biological indicators (phosphate solubilizing bacteria, phosphate solubilizing fungi, cellulolytic bacteria, cellulolytic fungi, and nitrogen fixing bacteria) show the diversity and abundance in soil components based on factors such as the presence of micro and macroorganisms, bacteria, earthworms, and soil fungi (Bautista et al., 2004).
Another important indicator is soil enzymes, which are involved in microorganism activity. Together with other soil organisms, they are linked to energy transfer, biochemical processes, and environmental quality. Additionally, they are considered to be an important indicator of soil fertility (providing adequate amounts of nutrients) and agronomic, ecological, and anthropogenic influence (Doran & Parkin, 1994).
Finally, despite the importance of EA techniques for soils, there are still not enough studies showing possible changes in soil quality indicators as a result of the application of said techniques over time, specifically in coffee plantations under different coverings. For this reason, the main question of this research is: what changes occur in the soil quality indicators of coffee plantations under different coverings, which are associated with the implementation of agriculture ecological techniques in the municipality of San José de Pare, Boyacá?
Materials and methods
Description of the study area
This research was carried out in a rural area located in the Colombian municipality of San José de Pare, in the department of Boyacá, at an altitude of 1545 m.a.s.l. and average temperatures of 13 and 24 ºC, with the following geographical coordinates: 6º01.84' N longitude, 73º31' W latitude. This municipality is located in the Republic of Colombia, 217 km north of Bogotá DC, in the northeastern region of the department of Boyacá, and it shares borders with the municipalities of Chitaraque, Moniquirá, San José de Pare, Santana, and Togüi.
Experimental design and soil sampling
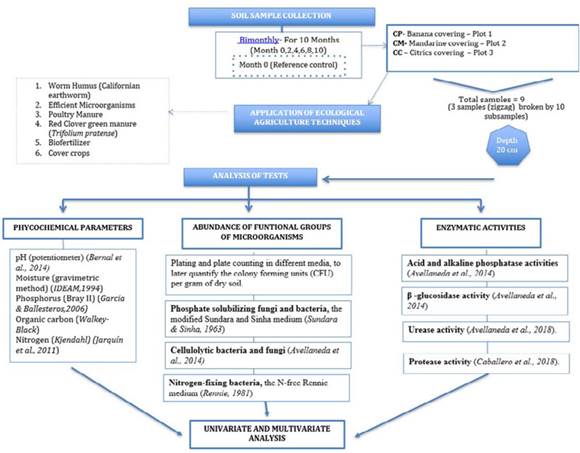
As shown in Figure 1, sampling was carried out by selecting three coffee plots (twelve coffee plants in each plot) under three different coverings: banana trees (CP), mandarin trees (CM), and three citrus fruit trees (CC) (mandarin, lemon, and orange). The EA techniques applied were: 1) worm humus (Californian earthworm) (Milanés et al., 2005); 2) biofertilizer, obtained from the mixture of legumes, molasses, yeast, chicha, poultry manure, rabbit manure, wood ash, and milk (Trejo-Escareño et al., 2013); 3) efficient microorganisms (EM) from a mix of leaf litter, rice grits, molasses, and milk (Callisaya & Fernández, 2017); 4) poultry manure (González et al., 2013); 5) Red clover green manures (Trifolium pratense) (Fonseca-López et al., 2020); and 6) cover crops (Geissert et al., 2017). As the EA techniques were being applied, soil samples were collected on a bimonthly basis (months 0, 2, 4, 6, 8, 10) for ten months, for a total of 6 sampling moments. Each plot provided three samples composed of 10 different subsamples, which were taken in zigzag from the upper 20 cm of the soil. The soil samples were then homogenized, labeled, and stored in bags at different temperatures depending on their purpose: the bags for physicochemical analysis at room temperature, those for microorganism abundance analysis at 4 °C, and those for enzymatic activities at -20 °C. The samples were processed in the shortest possible time after sampling (maximum one week after collection).
Figure 1: Methodology for the application of EA techniques from soil sample collection
Physicochemical parameters of the soil
All samples underwent physicochemical analysis according to the following methods:
-
pH (potentiometer): 1:1, soil:water. (Bernal et al., 2014).
-
Moisture (gravimetric method): it consists of taking a soil sample, weighing it before and after drying, and calculating its moisture content. The soil sample is considered to be dry when its weight remains constant at a temperature of 105 ºC (IDEAM, 1994).
-
Phosphorus (Bray II): the soil phosphorus is extracted with 25 ml of the extractor solution of HCl 0.1N and NH4F 0.03N, shaken, filtered, and the available phosphorus is colorimetrically quantified by reaction with chloromolybdic acid and stannous chloride to form a blue complex whose intensity is proportional to the amount of the element present (García & Ballesteros, 2006).
-
Organic carbon (Walkley-Black): this method is based on the oxidation of the organic carbon in the soil by means of a solution of potassium dichromate (K2Cr2O4) and the heat of the reaction generated by mixing with concentra-ted sulfuric acid (H2SO4). After a waiting time, the mixture is diluted, phosphoric acid is added to avoid Fe3+ interferen-ces, and the residual potassium dichromate is titrated with ferrous sulfate (FeSO4 1N. pH 7) (Vela et al., 2012).
-
Nitrogen (Kjendahl): which consists of three stages digestion, distillation and titration, where the sample is left in digestion with pure sulfuric acid, then an absorbent solution such as boric acid is used for the distillation stage and finally a titration with 1N sulfuric acid is performed (Jarquín et al., 2011).
Abundance of functional groups of microorganisms
To determine the abundance of functional microorganism groups, we performed plating and plate counting in different media, to later quantify the colony forming units (CFU) per gram of dry soil. For the seeding and counting of phosphate solubilizing fungi and bacteria, we used the modified Sundara and Sinha medium (Sundara & Sinha, 1963). The procedure was carried out by incubating the bacteria at 28 ºC for 40 h and the fungi at 20 ºC for six days; for cellulolytic bacteria and fungi, we used a medium whose only source of carbon was 1% carboxymethyl cellulose, where bacteria were incubated at 28 ºC for 48 h and fungi at 20 ºC for six days (Avellaneda-Torres et al., 2014); and, finally, for the nitrogen-fixing bacteria, we used the N-free Rennie medium (Rennie, 1981) and CFU plate count by maintaining the temperature at 28 ºC for 48 h (Avellaneda-Torres et al., 2020).
Enzymatic activity
With the purpose of evaluating the enzymatic activities, we considered the association with phosphorus, carbon, and nitrogen cycles. For the phosphorus cycle, the study determined acid and alkaline phosphatase activities, which were quantified by determining the p-nitrophenol that was released after the incubation with p-nitrophenyl phosphate solution for 1 h at 37 ºC. The p-nitrophenol was photometrically determined at 400 nm. For the carbon cycle, the β-glucosidase activity was determined by quantifying the p-nitrophenol released after the incubation with a p-nitrophenyl glucoside solution for 1 h, at 37 ºC. The p-nitrophenol was photometrically determined at 400 nm. As for the nitrogen cycle, the urease activity was determined, which is based on the colorimetric determination at 690 nm of the released ammonia after incubation with a solution of urea for 2 h, at 37 ºC (Avellaneda-Torres et al., 2018). Finally, the study determined the protease activity by incubating the soil samples for 2 h at 50 ºC using casein as substrate (Caballero-Vanegas et al., 2018) after incubation for 2 h at 50 ºC and a pH 8.1. In this method, the released amino acids reacted with the Folin-Ciocalteu reagent in an alkaline solution to form a blue complex that was colorimetrically evaluated (700 nm) (Avellaneda-Torres et al., 2018).
Statistical analysis
To determine if there were significant differences in the physicochemical parameters, the abundance of functional groups of microorganisms, and the enzymatic activities throughout the months of application of EA techniques in the soils with coffee plantations under three different coverings (CP, CM, and CC), we carried out univariate and multivariate data analyses. To this effect, the assumptions of normality and homogeneity of variances were verified using the Kolmogorov-Smirnov and Barlett tests, respectively, followed by the Kruskal-Wallis tests with a post-hoc Mann-Whitney analysis to determine significant differences. The tests were performed with the Stats package (R Core Team, 2019). A principal component analysis (PCA) was also performed using the factoextra package (Kassambara & Mundt, 2020). The analyses were performed with the R statistical software, version 3.5.3. (R Core Team, 2019).
Results
Physicochemical parameters
According to the results presented in Table 1, it appears that, on average, we can find a 29.3% for CP in the Ɵg, which shows that it is a soil with good moisture content, as well as an average of 37.4 and 48.7% for CM and CC, respectively. This means that, according to its water content, it is a usable soil. In general, the pH in registers strongly acid values between (5.0 and 5.7), thus indicating that some of the elements that are available in the soil do not have adequate levels. On the other hand, we found an average CEC of 26.4 meq.100g-1 for CP and 38.7 meq.100g-1 for CM, which indicates that it is a medium CEC soil, whereas 41.4 meq.100g-1 for CC imply a high CEC soil. Regarding the phosphorus content, an average of 30.6% was obtained for the three plots, indicating that phosphorus in the soil under study is at high levels. It was observed that carbon levels were high, as they ranged between 4.3 and 8.7%. Finally, nitrogen content values were high (between 0.4 and 0.9%).
Table 1: Physicochemical parameters by coverings and time of application of EA techniques in months: gravimetric moisture (Ɵg), pH, cation exchange capacity (CEC) in meq.100g-1, phosphorus (P) in ppm, organic carbon (OC) in %, and nitrogen (N) in %. Different letters indicate statistically significant differences, whereas equal letters symbolize no significant difference (p<0.05)
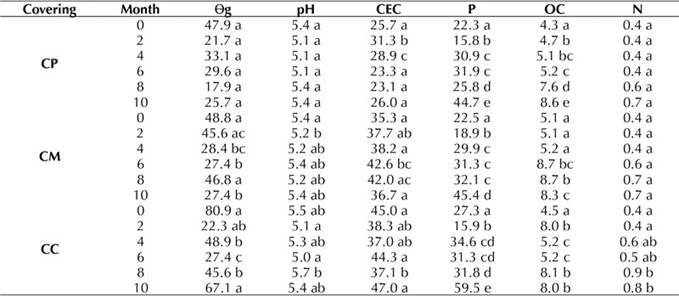
According to Table 1, the Ɵg shows fluctuations throughout the months with each of the coverings. However, they are not statistically significant. Moreover, the pH of the soils did not change significantly for the three evaluated coverings as the months EA technique application elapsed. In addition, there were no significant changes in the CEC during this period. For organic carbon, a significant increase was observed in the three coverings. The same behavior was seen in the case of phosphorus, where a significant increase was evident in each of the coverings, with the highest statistical increase in month 10 for both phosphorus and carbon. There was no significant change for nitrogen over the months of application of the EA techniques.
Functional groups of microorganisms
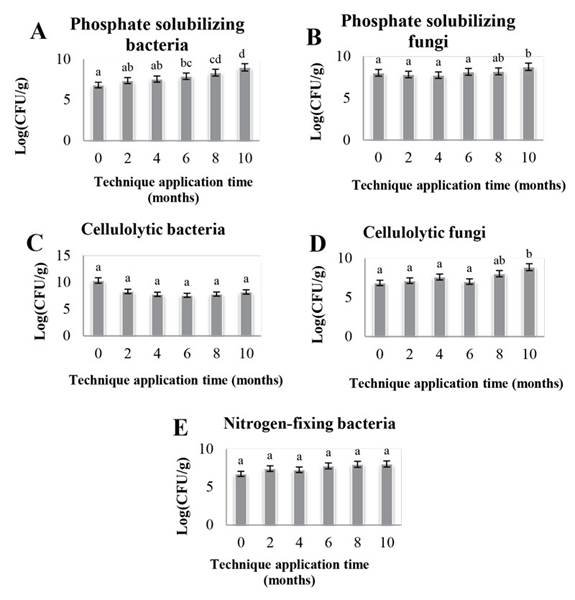
The results for the abundance of functional groups of microorganisms show a statistically significant increase as the months of application of EA techniques elapsed in the case of phosphate solubilizing bacteria (Figure 2a). Phosphate solubilizing fungi (Figure 2b) show a significant increase, whereas cellulolytic bacteria (Figure 2c) did not show statistically significant changes during this period. Cellulolytic fungi (Figure 2d) increased statistically during the last months of study, and, finally, nitrogen-fixing bacteria (Figure 2e) did not show any significant changes.
Figure 2: Abundance of functional groups of microorganisms in counts for CP, CM, and CC. Different letters indicate statistically significant difference; equal letters symbolize no statistically significant difference p<0.05
Enzymatic activity
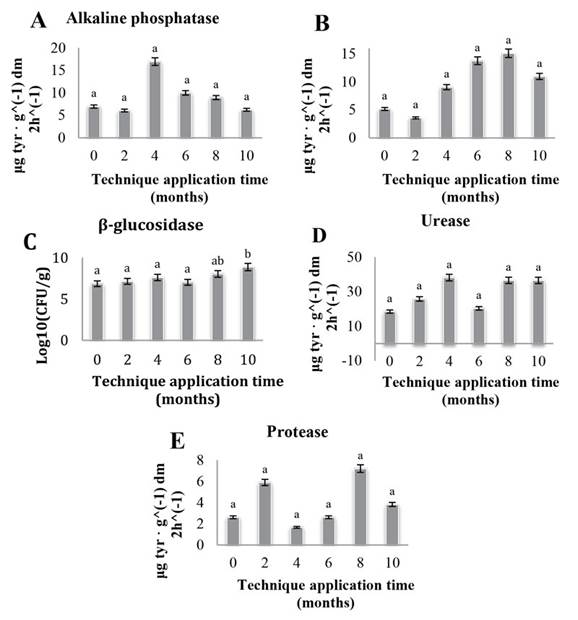
Figure 3 shows that there is no significant change in the enzymatic activity corresponding to alkaline phosphatase (Figure 3a), acid phosphatase (Figure 3b), β-glucosidase (Figure 3c), urease (Figure 3d), and protease (Figure 3e) during the months of application of the EA techniques. On the other hand, the statistical analyses for enzymes in each crop (banana, mandarin, and citrus) accompanying the coffee plantations showed the same results, which is why they are not presented in this paper.
Figure 3: Average CP, CM, and CC enzymatic activity. Different letters indicate significant statistical difference, equal letters symbolize no significant statistical difference p<0.05
Principal component analysis of different variables in response to EA techniques
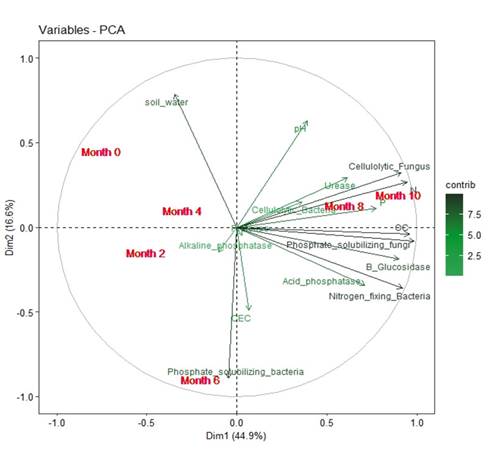
The cumulative variance of the PCA in (Figure 4) was 60.2%, which constitutes an indicator of good quality in the analysis of the results. It can be observed that, towards the last two months of sampling and application of EA techniques, there is a correlation between the variables of phosphorus, organic carbon, phosphate solubilizing fungi, phosphate solubilizing bacteria, and cellulolytic fungi. pH, nitrogen, and enzymatic activities such as β-glucosidase and urease appear in a lower proportion. The behavior of these variables is opposite to that observed for gravimetric moisture, showing that the lower the soil moisture with respect to the months of application, the higher the percentages of the different variables opposite to it. Likewise, another inversely proportional relationship takes place between pH and phosphate solubilizing bacteria, which indicates that the count of these bacteria depends on the change in soil pH.
Figure 4: Principal component analysis of the parameters for the three plots from month 0 to month 10 of application of the EA techniques. CEC: cation exchange capacity, P: phosphorus, OC: organic carbon, and N: nitrogen.
Component 1 has a variance of 60%, and, together with component 2 of variance 40, they represent 100% of the total variation.
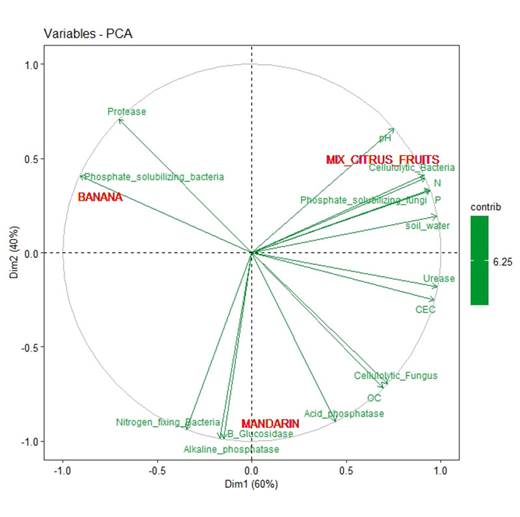
Figure 5 shows that the accumulated variance of the PCA was 100%, which indicates a low stress analysis for the understanding of the variables. It is observed that CP is correlated with the protease and phosphate solubilizing bacteria. CM shows a correlation with variables such as organic carbon, CEC, cellulolytic fungi, nitrogen-fixing bacteria, acid and alkaline phosphatase, and β-glucosidase. Finally, there is a higher correlation for CC among variables such as gravimetric moisture, pH, phosphorus, nitrogen, microorganisms, phosphate solubilizing fungi, and cellulolytic carbon bacteria, as well as for enzymes such as urease.
Component 1 has a variance of 9.6 and groups 60% of the variation, and, together with component 2 of variance 6.4, which groups 40% of the variation, they represent 100% of the total variation.
Figure 5: Principal component analysis of the three coverings (CP, CM, and CC)
Discussion
This research focused on evaluating whether the application of EA techniques generates significant changes in soil quality indicators in coffee plantations with different covering crops (CP, CM, and CC). It was found that variables such as phosphorus, organic carbon, phosphate solubilizing bacteria, phosphate solubilizing fungi, and soil cellulolytic fungi increased significantly during the months of application of the EA techniques, namely, vermicomposting, liquid humus, biofertilizer, EM, green manures, strengthening of polycultures, and composted poultry manure.
Said applications of EA techniques, together with agroforestry systems, integrate practices in which a woody component (tree or shrub) is combined with an agricultural one, thus allowing an improvement in the soils with respect to the production per unit area. In other words, it enhances productivity, thus obtaining more than a single product from the same soil unit (Henao, 2020) and promoting polyculture which is one of the practices of applied EA techniques.
The increase in phosphorus may be due to the enrichment of organic compounds from biofertilizers, which can improve phosphorus dynamics, resulting in increased nutrient availability (Rosatto et al., 2014).
In contrast, the increase in soil organic carbon may be due to the application of green manure and humus techniques, since they are carbon-rich, which allows a greater biointensity of the soil (Martínez et al., 2008). Likewise, this increase is linked to an important component such as humus, which can represent up to 90% of the total carbon in the soil (Jaramillo, 2011), meaning that adding organic matter to the soil makes the incorporation of vermicompost stand out due to its high stability, high content of bacterial fiber, and high content of nutrients that can be assimilated by plants (Salinas-Vásquez et al., 2014).
Furthermore, the use of EA techniques promotes the processes of some microorganisms that can synthesize organic acids, releasing phosphate ions from the exchange surface, thus increasing the concentration of these ions in the soil solution and therefore increasing their availability (Tapia & García, 2013). Additionally, the organic amendments used could cause an increase in soil phosphorus due to their derived composition (water, legumes, yeast, cow, and rabbit manure), given that they are associated with an increase in organic matter, which stores soil nutrients that improve its structure, as well as with the concentration of phosphorus in the soil, as plant roots absorb phosphorus (Múnera, 2014). Regarding biological indicators, the results obtained showed statistical increases in phosphate solubilizing bacteria and fungi, as well as in cellulolytic fungi. These results coincide with those found by other studies that evaluated the effect of the application of mycorrhizae in coffee growing systems (Avila et al., 2020).
Many soil microorganisms can transform insoluble phosphorus into forms that can be assimilated by plants, such as phosphate solubilizing bacteria. The increase in such bacteria may be related to the ionic forms of Pi (primary soil minerals) such as the H2PO4- ion, which can be absorbed by plants due to its solubility in water. These are dependent on pH, which ranges between 4.0 and 6.0 (Restrepo et al., 2015). Considering that the pH of this study was between 5.1 and 5.6, carrying out these processes is considered feasible. Likewise, this is correlated with the increase in inorganic phosphorus in the soil, which may be associated with a greater number of colony-forming units of phosphate solubilizing microorganisms as the application of EA techniques for the purposes of this study progressed.
As for phosphate solubilizing fungi, a significant increase was evidenced before the application of the EA techniques, mainly with the biofertilizer, which yielded positive responses for crops (Patiño & Sanclemente, 2014). These microorganisms transform insoluble mineral compounds into soluble minerals that are absorbed by plants (phosphorus solubilization) (Alejo-Iturvide et al., 2016).
Additionally, we observed a significant increase in cellulolytic fungi, possibly because of the increase in organic matter related to the application of the EA techniques, which promote an increase in cellulolytic microorganisms, and the presence of cellulose as a source for carbon, since this is the major component of plant biomass found in organic matter (Monserrate et al., 2014). These results match those found by other authors when comparing organic farming systems in coffee with conventional ones (Garzón et al., 2020). Additionally, efficient microorganisms (EM) secrete beneficial substances such as vitamins, organic acids, antioxidants, among others. These antioxidants promote the decomposition of organic matter and increase the content of humus in the soil (Liriano et al., 2015).
As for soil enzymatic activities, in general, it can be observed that there was no significant increase related to the application of EA techniques. In the case of acid and alkaline phosphatase, the absence of significant changes during the develop- ment of this research may be due to the fact that a high P content can inhibit the action mechanism of the enzymes by having a greater availability of nutrients in the soil in labile form (Narváez et al., 2010). The β-glucosidase enzyme, in turn, is one of the most abundant and important enzymes for soil quality. However, no significant change was found, which may be due to the fact that, in order to demonstrate its changes, longer times are required for the application of EA techniques (Gutiérrez et al., 2008).
On the other hand, regarding the covering crops that accompany the coffee crop (CP, CM, and CC), and as it can be observed in Figure 5 for ACP, the nitrogen-fixing bacteria, acid phosphatase, alkaline phosphatase, and β-glucosidase increased for CP. For CC, the pH, phosphorus, nitrogen, phosphate solubilizing fungi, cellulolytic bacteria, and urease also increased. The same behavior was observed for organic carbon, cellulolytic fungi, and acid phosphatase with both CC and CM. This has to do with the relationships between the increase in organic matter and the application of techniques such as vermicomposts, biofertilizers, efficient microorganisms, and green manures. This coincides with reports that soil coverings contribute to the stimulation and optimization of soil biological processes, improving soil fertility, counteracting erosion processes, and favoring the presence of populations of beneficial organisms (Puertas et al., 2008).
Moreover, it can be seen in Figure 4 (PCA) that there is a direct correlation for months 8 and 10 with nine of the variables that were analyzed, namely phosphorus, organic carbon, nitrogen, cellulolytic fungi, nitrogen-fixing bacteria, phosphate solubilizing fungi, and β-glucosidase, which are variables that increased due to the application of EA techniques because they generated a high content of organic matter and high biological activity in soils, thus highlighting the importance of applying these methods over time (Gutiérrez V. et al., 2008).
Conclusions
According to the univariate data analysis of the six variables evaluated on physicochemical properties, a significant increase in the phosphorus and organic carbon was reported in the coffee soils under study. It was also observed that, due to the application of the EA techniques, the five functional groups of quantified microorganisms showed a significant increase in the abundance of phosphate solubilizing bacteria and fungi, as well as cellulolytic fungi.
As for the multivariate data analysis, the PCA determined that, during months 8 and 10 of application of the EA techniques, there was a significant increase in the content of phosphorus, organic carbon, nitrogen, the abundance of cellulolytic fungi, nitrogen-fixing bacteria, phosphate solubilizing fungi, and β-glucosidase.
Acknowledgements
Acknowledgments
We would like to thank Mrs. Angelica Rivera and Mr. Flaminio Gomez for allowing us to conduct this study in their coffee plantations. We also acknowledge all those who enabled the execution of the project in the area.
References
Licencia
Derechos de autor 2022 Colombia forestal

Esta obra está bajo una licencia internacional Creative Commons Atribución-CompartirIgual 4.0.
Colombia Forestal conserva los derechos patrimoniales (copyright) de las obras publicadas, y favorece y permite la reutilización de las mismas bajo la licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional por lo cual se pueden copiar, usar, difundir, transmitir y exponer públicamente, siempre que:
Se reconozcan los créditos de la obra de la manera especificada por el autor o el licenciante (pero no de una manera que sugiera que tiene su apoyo o que apoyan el uso que hace de su obra).