DOI:
https://doi.org/10.14483/2256201X.19114Publicado:
01-01-2023Número:
Vol. 26 Núm. 1 (2023): Enero-junioSección:
Artículos de investigación científica y tecnológicaMicropropagation and Germplasm Conservation of Ficus americana Aubl. and F. obtusifolia Kunth from Lambayeque (Peru)
Micropropagación y conservación de germoplasma de Ficus americana Aubl. y F. obtusifolia Kunth de Lambayeque (Perú)
Palabras clave:
acclimatization and survival, Moraceae, Piper culture medium, seasonally dry tropical forests, seed germination (en).Palabras clave:
aclimatación y supervivencia, Moraceae, medio de cultivo de Piper, bosque tropical estacionalmente seco, germinación de semillas (es).Descargas
Referencias
Ahmed, H., Khan, M. Z. I., Waseem, D., Nazli, A., & Baig, M. W. (2017). Phytochemical analysis and antioxidant potential of Ficus benghalensis L. Journal of Bioresource Management, 4(3), 17-26. http://doi.org/10.35691/JBM.7102.0075 DOI: https://doi.org/10.35691/JBM.7102.0075
Al-Shomali, I., Sadder, M. T., & Ateyyeha, A. (2017). Culture media comparative assessment of common fig (Ficus carica L.) and carryover effect. Jordan Journal of Biological Sciences, 10(1), 13-18. https://jjbs.hu.edu.jo/files/v10n1/Paper%20number%203.pdf
Andrade, C., Ferreres, F., Gomes, N. G. M., Duangsrisai, S., Srisombat, N., Vajrodaya, S., Pereira, D. M., Gil-Izquierdo, A., Andrade, P. B., & Valentão, P. (2019). Phenolic profiling and biological potential of Ficus curtipes corner leaves and stem bark: 5-lipoxygenase inhibition and interference with NO levels in LPS-stimulated RAW 264.7 macrophages. Biomolecules, 9(9), 400. http://doi.org/10.3390/biom9090400 DOI: https://doi.org/10.3390/biom9090400
Angiosperm Phylogeny Group (APG IV) (2016). An update of the Angiosperms phylogeny group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society, 181(1), 1-20.
http://doi.org/10.1111/boj.12385 DOI: https://doi.org/10.1111/boj.12385
Anjam, K., Khadivi-Khub, A., & Sarkhosh, A. (2017). The potential of caprifig genotypes for sheltering Blastophaga psenes L. for caprification of edible figs. Erwerbs-Obstbau, 59(1), 45-49. http://doi.org/10.1007/s10341-016-0296-4 DOI: https://doi.org/10.1007/s10341-016-0296-4
Babu, A., Anand, D., & Saravanan, P. (2017). Phytochemical analysis of Ficus arnottiana (Miq.) Miq. leaf extract using GC-MS analysis. International Journal of Pharmacognosy and Phytochemical Research, 9(6), 775-779. http://doi.org/10.25258/phyto.v9i6.8177 DOI: https://doi.org/10.25258/phyto.v9i6.8177
Bagyalakshmi, B., Nivedhitha, P., & Balamurugan, A. (2019). Studies on phytochemical analysis, antioxidant and antibacterial activity of Ficus racemosa L. leaf and fruit extracts against wound pathogens. Vegetos, 32, 58-63. http://doi.org/10.1007/S42535-019-00007-6 DOI: https://doi.org/10.1007/s42535-019-00007-6
Bayoudh, Ch., Labidi, R., Majdoub, A., & Mars, M. (2015). In vitro propagation of caprifig and female fig varieties (Ficus carica L.) from shoot-tips. Journal of Agriculture, Science and Technology, 17(6), 1597-1608. https://jast.modares.ac.ir/browse.php?a_code=A-23-1000-1610&slc_lang=en&sid=23
Bertaccini, G. (1982). Substance P. In G. Bertaccini (Ed.), Handbook of Experimental Pharmacology (vol. 59, pp. 85-105). Springer. DOI: https://doi.org/10.1007/978-3-642-68474-6_3
Boliani, A. C., Ferreira, A. F. A., Monteiro, L. N. H., da Silva, M. S. C., & Rombola, A. D. (2019). Advances in propagation of Ficus carica L. Revista Brasileira de Fruticultura, 41(3), e-026. http://doi.org/10.1590/0100-29452019026 DOI: https://doi.org/10.1590/0100-29452019026
Brako, L., & Zarucchi J. (1993). Catalogue of the flowering plants and gymnosperms of Peru. Missouri Botanical Garden.
Cáceres, B. P., & Reynel, C. (2010). Los árboles de Ficus (Ojé) del valle de Chanchamayo. Dp. de Junín, Perú (800-2500 msnm). Asociación Peruana para la Promoción del Desarrollo Sostenible (APRODES).
Corel Daw (2019). Corel DRAW Graphics Suite for Windows. Corel Corporation.
Datwyler, S. L., & Weiblen, G. D. (2004). On the origin of the fig: phylogenetic relationships of Moraceae from ndhF sequences. American Journal of Botany, 91(5), 767-777. http://doi.org/10.3732/ajb.91.5.767 DOI: https://doi.org/10.3732/ajb.91.5.767
Delgado-Paredes, G. E., Kato, M. J., Vásquez-Dueñas, N., Minchala-Patiño J., & Rojas-Idrogo, C. (2012). Cultivo de tejidos de Piper sp. (Piperaceae): propagación, organogénesis y conservación de germoplasma in vitro. Revista Colombiana de Biotecnología, 14(2), 49-60. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-34752012000200006
Delgado-Paredes, G. E., Duque-Aurazo, A. J., Vásquez-Díaz, C., & Rojas-Idrogo, C. (2017). Propagación masiva del matico (Piper tuberculatum Jacq.) y su aplicación en la erradicación de vectores de enfermedades metaxénicas en Lambayeque (Perú). Revista Latinoamericana de Recursos Naturales, 13(2), 39-50. https://www.itson.mx/publicaciones/rlrn/Documents/v13-n2-Propagaci%C3%B3n-masiva-del-matico-%28Piper-tuberculatum-Jacq.%29-y-su-aplicaci%C3%B3n-en-la-erradicaci%C3%B3n-de-vectores-de-enfermedades-metax%C3%A9nicas-en-Lambayeque-%28Per%C3%BA%29.pdf
Delgado-Paredes, G. E., Huamán-Mera, A., & Rojas-Idrogo, C. (2020a). Seasonally dry tropical forests of Peru: Current status and conservation with social responsability. Agricultural Research & Technology, 24(3), 556269. http://doi.org/10.19080/ARTOAJ.2020.24.5562659
Delgado-Paredes, G. E., Vásquez-Díaz, C., Tesén-Núñez, F., Esquerre- Ibañez, B., Zuñe Da-Silva, F., & Rojas-Idrogo, C. (2020b). Arboreal vegetation of the Cerro Tres Puntas de Pilasca, (Salas-Motupe), Lambayeque, Perú. Revista Mexicana de Ciencias Forestales, 11(58), 549. http://doi.org/10.29298/rmcf.v11i58.549 DOI: https://doi.org/10.29298/rmcf.v11i58.549
Delgado-Paredes, G. E., Vásquez-Díaz, C., Esquerre- Ibañez, B., Bazán-Sernaqué, P., Rojas-Idrogo, C. (2021). In vitro tissue culture in plants propagation and germplasm conservation of economically important species in Peru. Scientia Agropecuaria, 12(3), 337-349. http://doi.org/10.29298/rmcf.v11i58.549 DOI: https://doi.org/10.17268/sci.agropecu.2021.037
Duque-Aurazo, Y. A., Rojas-Idrogo, C., & Delgado-Paredes, G. E. (2020). In vitro clonal propagation, callus induction and germplasm conservation of ‘cerecillo’ Muntingia calabura L. (Muntingiaceae). International Journal of Research – GRANTHAALAYAH, 8(5), 277-289. http://doi.org/10.29121/granthaalayah.v8i5.2020.197 DOI: https://doi.org/10.29121/granthaalayah.v8.i5.2020.197
Engelmann, F. (1997). In vitro conservation methods. In J. A. Callow, B. Ford-Lloyd, H. J. Newbury (Eds.), Biotechnology and Plant Genetic Resources: Conservation and Use (vol. 19, pp. 119-191). CAB International.
Flores-Meza, M. M., Rojas-Idrogo, C., & Delgado-Paredes, G. E. (2014). Propagación clonal, conservación y transferencia internacional de germoplasma de yuca (Manihot esculenta Crantz) in vitro, nativas del Perú. Revista Latinoamericana de Recursos Naturales, 10(2), 32-44. https://revista.itson.edu.mx/index.php/rlrn/article/view/230/166
Ghanbari, A., Le Gresley, A., Naughton, D., Kuhnert, N., Sirbu, D., & Ashrafi, G. H. (2019). Biological activities of Ficus carica latex for potential therapeutics in Human Papillomavirus (HPV) related cervical cancers. Scientific Reports, 9(1), 1013. http://doi.org/10.1038/s41598-018-37665-6 DOI: https://doi.org/10.1038/s41598-018-37665-6
Gopukumar, S. T., Princy, A., Jainamboo, M., & Praseetha, P. K. (2016). Phytochemical screening and FT-IR analysis of Ficus benghalensis fruits. International Journal of Pharmacognosy and Phytochemical Research, 8(9), 1529-1534. http://impactfactor.org/PDF/IJPPR/8/IJPPR,Vol8,Issue9,Article16.pdf
Hassan, A. K. M. S., Afroz, F., Jahan, M. A. A., & Khatun, R. (2009). In vitro regeneration through apical and axillary shoot proliferation of Ficus religiosa L.-A multipurpose woody medicinal plant. Plant Tissue Culture Biotechnology, 19(1), 71-78. http://doi.org/10.3329/ptcb.v19i1.4987 DOI: https://doi.org/10.3329/ptcb.v19i1.4987
Haynes, W. (2013). Tukey’s Test. In W. Dubitzky, O. Wolkenhauer, K. H. Cho, & H. Yokota (Eds.), Encyclopedia of Systems Biology. Springer.
https://doi.org/10.1007/978-1-4419-9863-7_1212 DOI: https://doi.org/10.1007/978-1-4419-9863-7_1212
Hesami, M., Daneshvar, M. H., Yoosefzadeh-Najafabadi, M., & Alizadeh, M. (2018). Effect of plant growth regulators on indirect shoot organogenesis of Ficus religiosa through seedling derived petiole segments. Journal of Genetic Engineering and Biotechnology, 16(1), 175-180.
http://doi.org/10.1016/j.jgeb.2017.11.001 DOI: https://doi.org/10.1016/j.jgeb.2017.11.001
Ibarra-Manríquez, G., Cornejo-Tenorio, G., Gonzáles-Castañeda, N., Piedra- Malagón, E., & Luna, A. (2012). El género Ficus L. (Moraceae) en México. Botanical Sciences, 90(4), 389-452. http://doi.org/10.17129/botsci.472 DOI: https://doi.org/10.17129/botsci.472
Kornova, K. M., & Popov, S. K. (2009). Effect of the in vitro containers type on growth characteristics of the microplants in in vitro propagation of GF 677. Acta Horticulturae, 825(825), 277-282. http://doi.org/10.17660/actaHortic.2009.825.44 DOI: https://doi.org/10.17660/ActaHortic.2009.825.44
Kristiansen, K. (1991). Post-propagation growth of cuttings from in vitro and in vivo propagated stock plants of Ficus benjamina. Scientia Horticulturae, 46(3-4), 315-322. http://doi.org/10.1016/0304-4238(91)90054-3 DOI: https://doi.org/10.1016/0304-4238(91)90054-3
Kristiansen, K. (1992). Micropropagation of Ficus benjamina clones. Plant Cell, Tissue and Organ Culture, 28(1), 53-58. http://doi.org/10.1007/BF00039915 DOI: https://doi.org/10.1007/BF00039915
Lansky, E. P., & Paavilainen, H. M. (2010). Figs: The genus Ficus (1st ed.). CRC Press. DOI: https://doi.org/10.1201/9781420089677
Lavanya, D., Ghive, V. D., & Rao, N. G. V. (2006). In vitro multiplication of Ficus benjamina cv. Starlight. International Journal of Plant Sciences, 1(2), 315-317. http://researchjournal.co.in/online/IJPS/IJPS%201(2)/1_A-315-317.pdf
Machado, C. A., Robbins, N., Gilbert, M. T. P., & Herre, E. A. (2005). Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proceedings of the National Academy of Sciences of the United States of America, 102(suppl. 1), 6558-6565.http://doi.org/10.1073/pnas.0501840102 DOI: https://doi.org/10.1073/pnas.0501840102
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassy with tobacco tissue cultures. Physiologie Plantarum, 15, 473-497. http://doi.org/10.1111/j.1399-3054.1962.tb08052.x DOI: https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Patah, F. K. A., Hasbullah, N. A., Idris, H., Radzuan, N. S., & Mohammad, M. L. (2018, August 6-8). Micropropagation of Ficus carica L. through tissue culture system [Conference presentation]. International Conference on Advances in Agricultural Chemical, Biological & Medicinal Sciences (AACBMS-18), Pattaya, Thailand. http://doi.org/10.17758/EARES3.C0818108 DOI: https://doi.org/10.17758/EARES3.C0818108
Rajasekharan, P. E., & Sahijram, L. (2015). In vitro conservation of plant germplasm. In B. Bahadur, M.V. Rajam, L. Sahijram, & K.V. Krishnamurthy (eds.) Plant Biology and Biotechnology: Vol. II. Plant genomics and Biotechnology.. (pp. 417-443). Springer verlag. http://doi.org/10.1007/978-81-322-2283 DOI: https://doi.org/10.1007/978-81-322-2283-5_22
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
Reed, B. M., Sarasan, V., Kane, M., Bunn, E., & Pence, V. C. (2011). Biodiversity conservation and conservation biotechnology tools. In Vitro Cellular & Developmental Biology – Plant, 47(1), 1-4. http://doi.org/10.1007/s11627-010-9337-0 DOI: https://doi.org/10.1007/s11627-010-9337-0
Roca, W. M. (1982). Cultivo de meristemas de yuca. Guía de estudios. Centro Internacional de Agricultura Tropical (CIAT).
Shinkafi, S. A., & Abdullahi, H. (2018). Antifungal activity and phytochemical analysis of Ficus sycomorus leaf extract on Malassezia glubosa. Advances in Plants & Agriculture Research, 8(6), 432-436. http://doi.org/10.15406/apar.2018.08.00362 DOI: https://doi.org/10.15406/apar.2018.08.00362
Shorthouse, D. P. (2021). SimpleMappr, an online tool to produce publication-quality point maps. https://www.simplemappr.net/
Singh, B. M., Rajoriya, C. M., Wani, I. A., Rawat, R. S., & Jat, B. L. (2016). In vitro studies of Ficus carica and its application in crop improvement. International Journal for Research in Applied Science & Engineering Technology, 4(1), 135-148. https://www.ijraset.com/fileserve.php?FID=5774 1
Siwach, P., & Gill, A. R. (2011). Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiology and Molecular Biology of Plant, 17(3), 271-280. http://doi.org/10.1007/s12298-011-0074-6 DOI: https://doi.org/10.1007/s12298-011-0074-6
Siwach, P., Gill, A. R., & Kumari, K. (2011). Effect of season explants growth regulators, and sugar level on induction and long term maintenance of callus cultures of Ficus religiosa L. African Journal of Biotechnology, 10(24), 4879-4886. http://doi.org/10.58897/AJB10.2119
Siwach, P., & Gill A. R. (2014). Micropropagation of Ficus religiosa L. via leaf explants and comparative evaluation of acetylcholinesterase inhibitory activity in the micropropagated and conventionally grown explants. Biotech, 4(5), 477-491. http://doi.org/10.1007/s13205-013-0175-8 DOI: https://doi.org/10.1007/s13205-013-0175-8
Trejgell, A., Kamińska, M., & Tretyn, A. (2015). In vitro slow growth storage of Senecio macrophyllus shoots. Acta Physiologiae Plantarum, 37, 234. http://doi.org/10.1007/s11738-015-1983-8 DOI: https://doi.org/10.1007/s11738-015-1983-8
Zhang, L.-F., Zhang, Z., Wang, X.-M., Gao, H.-Y., Tian, H.-Z., & Li, H.-Q. (2018). Molecular Phylogeny of the Ficus auriculata complex (Moraceae). Phytotaxa, 362(1), 39-54.
http://doi.org/10.11646/phytotaxa.362.1.3 DOI: https://doi.org/10.11646/phytotaxa.362.1.3
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Recibido: 19 de febrero de 2022; Aceptado: 29 de septiembre de 2022
Resumen
Ficus americana y F. obtusifolia, se encuentran entre las especies arbóreas más importantes de los Bosques Tropicales Estacionalmente Secos (BTES) por su condición de siempre verdes y sus altos niveles de biomasa. Sin embargo, el BTES de Lambayeque y el norte de Perú está disminuyendo enormemente debido al avance de la agricultura migratoria, la minería ilegal y la deforestación. El objetivo de este trabajo fue estudiar los aspectos taxonómicos de ambas especies, así como la germinación de semillas, la micropropagación y la conservación de germoplasma in vitro. La germinación de semillas fue de 100 % en ambas especies hasta tres meses después de la recolección. Respecto a la micropropagación, el enraizamiento y la conservación de germoplasma, el medio de cultivo de Piper resultó efectivo, el cual está conformado por sales minerales MS con IAA 0.02 mg.L-1 y GA3 0.02 mg.L-1. La conservación in vitro de germoplasma duró más de 24 meses en ambas especies. La aclimatación en condiciones de invernadero alcanzó 50 % de supervivencia en ambas especies.
Palabras clave:
aclimatación y supervivencia, Moraceae, medio de cultivo de Piper, bosque tropical estacionalmente seco, germinación de semillas..Abstract
Ficus americana and F. obtusifolia are among the most important tree species in Seasonally Dry Tropical Forests (SDTF) due to their evergreen condition and high levels of biomass. However, the SDTF of Lambayeque and northern Peru is greatly diminishing due to the advance of migratory agriculture, illegal mining, and deforestation. The objective of this work was to study the taxonomic aspects of both species, as well as seed germination, micropropagation, and in vitro germplasm conservation. Seed germination was 100% for both species up to three months after collection. As for micropropagation, rooting, and germplasm conservation, the Piper culture medium was effective, as it was constituted by MS mineral salts with 0.02 mg.L-1 IAA and 0.02 mg.L-1 GA3. In vitro germplasm conservation lasted more than 24 months for both species. Acclimatization under greenhouse conditions reached 50% survival for both species.
Keywords:
acclimatization and survival, Moraceae, Piper culture medium, seasonally dry tropical forests, seed germination..INTRODUCTION
Ficus americana Aubl. and F. obtusifolia Kunth are the only wild species of the genus Ficus in the Seasonally Dry Tropical Forest (SDTF) of the Lambayeque region (Peru). In the Angiosperm Phylogeny Group classification, the family Moraceae (to which Ficus belongs) is grouped into class "Rosids", in the order Rosales, together with the families Cannabaceae, Rhamnaceae, Rosaceae, Ulmaceae, Urticaceae, and others (APG IV, 2016). However, the taxonomy of the genus Ficus (Moraceae) and its c. 800 species (Lansky & Paavilainen, 2010) is very complex and, as with other groups of angiosperms, the use of several molecular markers has been necessary to fully understand the infragenic relation between the species. The nuclear ITS and G3pdh loci, several chloroplast loci, and 15 pairs of SSR markers have been used to aid the reconstruction of the phylogenetic relationship and clarify the species’ boundaries (Zhang et al., 2018). This has shown the distinct molecular phylogeny of the F. auriculata Lour. complex, namely a pronounced distinction between F. auriculata, F. oligodon Miq., F. hainanensis Merr. & Chun., F. beipeiensis S.S. Chang, and F. variegata Blume (Zhang et al., 2018).
The first studies on the micropropagation of Ficus species (Moraceae) were carried out in the commonly cultivated species F. benjamina L., observing that cuttings from in vitro propagated plants rooted faster, as well as with a higher rooting percentage, than cuttings grown using conventional methods for mass propagating large numbers of plants (Kristiansen,1991). A similar response was observed in the superior clone “Cleo” for eight subsequent subcultures (Kristiansen, 1992). Using axillary shoots in the same species (cv. Starlight), a high proliferation of shoots was achieved using an MS culture medium supplemented with 1.0 mg.L-1 BAP and 50 mg.L-1 adenine sulphate, while optimal rooting was observed with 1.0 mg.L-1 NAA and 1.0 mg.L-1 activated charcoal (Lavanya et al., 2006).
In vitro clonal propagation using leaf cuttings from 45-50 year-old adult plants was studied only in F. religiosa L. (Sacred Fig or Bo-Tree). F. religiosa is a tree species native to India and Nepal, and it is primarily known to be important in the treatment of Alzheimer’s disease (Bertaccini, 1982). It was observed that the highest frequency of response and the highest rate of shoots occurred using 5.0 mg.L-1 BAP (Siwach & Gill, 2014). Previous studies using F. religiosa have largely employed different tissues and elements, including apical and axillary buds, callus maintenance, culture medium supplemented with adenine sulphate, glutamine and phloroglucinol, and the season of simple collection. However, in all these studies, the contamination rate was significantly high and thus may not provide an accurate reading of the propagation potential (Hassan et al., 2009; Siwach & Gill 2011; Siwach et al., 2011).
The common fig tree, F. carica L., originally native to Western Asia but cultivated worldwide, is undoubtedly the most important food species within the genus Ficus. For this reason, several methods of sexual propagation have been developed (seedling production with entomophilic or vegetative pollination), as well as several conventional methods of multiplication such as asexual cuttings, plunging, grafting, and tissue culture techniques (Boliani et al., 2019). In four of the varieties of F. carica, in vitro propagation using shoot-tip (0.5 to 1.5 mm in length) cultivation in an MS culture medium supplemented with various combinations of plant growth hormones has shown particular efficacy for eliminating various viral diseases such as the Fig Mosaic Disease (FMD) (Bayoudh et al., 2015). Some techniques, such as using stem segments isolated from seedlings, micropropagation of F. carica using the MS culture medium supplemented with 1.0 mg.L-1 BAP, and callus induction from different cuttings with varying combinations of BAP-NAA (Patah et al., 2018), have shown to be among the best conventional methods for the micropropagation of F. carica. Various similar techniques developed for plant tissue cultures and their mass application have been described by Singh et al. (2016).
The phytochemical analysis of several Ficus species has revealed the presence of numerous secondary compounds of therapeutic importance. F. arnottiana (Miq.) Miq. has shown twenty-eight bioactive secondary metabolites in chloroform and ethanol extracts, highlighting tritetracontane and 2, 6-lutidine 3,5-dichloro-4-dodecylthio, with various degrees of pharmacological properties (Babu et al., 2017). In F. benghalensis L., commonly known as the Indian Banyan Tree, phytochemical analysis has shown the highest total concentrations of phenolic contents (TPC) and total flavonoid content (TFC) in ethyl acetate extracts of the stem and leaves, respectively. The total reduction power and total antioxidant capacity, however, were higher in the stem extract of this species (Ahmed et al., 2017). Furthermore, in the phytochemical screening of methanol, chloroformic, and aqueous fruit extracts using FT-IR spectroscopy, numerous secondary metabolites were detected. In particular, flavonoids, alkaloids, tannins, terpenoids, and phlobatanins were abundant in the methanol extract (Gopukumar et al., 2016). The phytochemical analysis of F. sycomorus L. leaves collected in Nigeria showed the presence of antifungal alkaloids, glycoside, tannins, flavonoids, and saponins (Shinkafi & Abdullahi, 2018).
Biological activity has been reported for several species of Ficus. Aqueous and varying concentrations (10 to 100 mg.mL-1) extracted from the leaves of F. sycomorus were tested against Malassezia globosa CBS 7966, a fungal species that can cause pathogenic skin diseases such as pityriasis and dermatitis under certain conditions (Shinkafi & Abdullahi, 2018). Latex was obtained from the unripe fruits of F. carica and has been studied for its potential in the therapeutic treatment of the human papillomavirus (HPV), which is related to cervical cancers such as CaSki and HeLa. There are three main isolated latex compounds that are active in the treatment of CaSki and HeLa: oleandiene, lanostariene, and stigmasterol (Ghanbari et al., 2019). In another study, various phytochemical characterization techniques were used, such as HPLC-DAD-ESI (Ion Trap)/MSn qualitative analysis, in order to obtain methanol extracts from the bark and leaves of F. curtipes Corner. This technique allows isolating and identifying flavan-3-ols, apigenin derivatives, and apigenin-di-glycosides. Both extracts inhibited 5-lipoxygenase (5-LOX) activity and interfered with nitric oxide (NO) levels in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages (Andrade et al., 2019). Similar studies involving the phytochemical analysis, antioxidant properties, and antibacterial activity of ethanol, toluene, and ethyl acetate extracts of F. racemosa L. leaves and fruits have been conducted, which have additionally determined the presence of alkaloids, flavonoids, and phenols with high antioxidant potential. In a similar manner, bacteria from the wounds of diabetic patients such as Staphylococcus spp., Pseudomonas spp., and Klebsiella spp. could be effectively treated, as they have shown significant inhibition following the application of fruit extracts (Bagyalakshmi et al., 2019).
The main objective of this research was to taxonomically classify the most frequent species of Ficus (Moraceae), F. americana, and F. obtusifolia present in the SDTF of the Lambayeque region in Peru. Seed germination, micropropagation, and in vitro germplasm conservation were studied in order to establish a broad program for reforestation, mass propagation, and conservation, with the purpose of partially mitigating the effects of climate change.
MATERIALS AND METHODS
Plant materials
Ripe fruits of both species, Ficus americana and F. obtusifolia, were collected in the field and kept under the best physiological and phytosanitary conditions for sterile preservation and limited chance of contamination in the temporary seed bank of the General Laboratory of Biotechnology (Universidad Nacional Pedro Ruiz Gallo, Lambayeque, Peru). Three fruits (syconium) were collected from three different trees by species and by locality, selecting the best seeds according to their size, shape, and color, by means of a stereo microscope (NINGBO Teaching Instrument Co. Ltd.). These accessions come from field collections of previously identified healthy and mature trees (30-50 years old) from localities in the Provinces of Chiclayo: Chiclayo (06°47’35.5” latitude S - 79°52’54” longitude W); and Lambayeque: Pilasca (06°14'14.6" latitude S - 79°35'34.1" longitude W), Kerguer (06°11'12.2" latitude S - 79°30'39.9" longitude W), Chóchope Antiguo (06° 08'54.6" latitude S - 79°38'29.1" longitude W), and Ferreñafe: Laquipampa (06°21'10.9" latitude S - 79°26'41" longitude W). These trees were growing in SDTF environments of the Lambayeque region in northern Peru (Figure 1).
Figure 1: Collection map of Ficus americana and F. obtusifolia in the Lambayeque region (Peru)
Taxonomic aspects
Morphological descriptions were based on herborized and turgid material, which was dissected, analyzed, and photographed for later identifications supplemented with the pre-existing literature, such as the work by Cáceres and Reynel (2010) and Ibarra-Manríquez et al. (2012), as well as by consulting specialists in the genus. Two LCDP (Lankester Composite Dissection Plates) were also elaborated to illustrate the morphological descriptions. The plates were produced in Corel Draw (2019) and Paint Shop Pro (2018), and the map was created using the Simplemappr online tool (Shorthouse, 2021).
Micropropagation and acclimatization
The fruits were washed with a conventional detergent and disinfected with Benlate 0.2% for 30 minutes. Then, they were dissected, and, with the help of a stereomicroscope, the largest seeds deemed to be in the best morphological conditions were selected. These seeds were additionally cleaned using a 25 nylon mesh and washed with a 70% ethanol solution for 1 minute. Commercial sodium hypochlorite (Clorox® with 5.25% active chlorine) containing 0.02% (v/v) Tween-20 was also applied for 10 minutes. Subsequently, the seeds were rinsed three consecutive times with sterilized distilled water in order to clear any residue. They were grown in numbers of 3 to 5 in a culture medium comprising MS minerals (Murashige & Skoog, 1962) supplemented with 2% sucrose and 0.7% agar-agar.
After 60-90 days, the seedlings were propagated by stem tips and nodal segments in an MS culture medium supplemented with 2% sucrose, 0.7% agar-agar, and various growth regulators, including auxins, cytokinins, and gibberellins.
During the acclimatization process, seedlings between 30 and 45 days old and 3-4 cm in size were used, which contained 2-4 leaves, 1-3 roots of 2-5 cm in length, and no basal callus formation. Sterilized farmland + vermiculite (2:1) was employed as substrate to idealize the growing conditions without external contamination of the samples.
Preparation of the culture medium and the environmental incubation conditions
Various formulations of the culture medium were prepared during the rooting phase. These media were supplemented with 1.0 mg.L-1 thiamine.HCl and 100 mg.L-1 m-inositol. Several 150 x 25 mm Petri dishes, 110 x 13 mm test tubes, and 80 x 60 cm glass flasks were used to maintain sterility depending on the phase of the experiments. Once stored, the pH of all culture media was adjusted to 5.8 ±0.1 with 0.1N HCl and 0.1 N KOH. They were sterilized in an autoclave at 125 lb/in2 pressure and a temperature of 121 oC for 20 minutes.
The propagation conditions of the cultures were 24 ±2 oC with a 16/8 h photoperiod at 50 μmol m-2s-2, which was provided by cool-white fluorescent lights and a 80-90% relative humidity. Each treatment comprised 15 repetitions and was performed twice in order to obtain the best average sampling rates.
Statistical analysis
To check for significant differences between the species, all attributes were subjected to an analysis of variance (ANOVA) and a Tukey test (Haynes, 2013) in the R 4.0.5 software (R Core Team, 2022).
RESULTS
Taxonomic aspects
Ficus americana Aubl. 1775
Collection site: Chóchope Antiguo (Chóchope, Lambayeque) and Laquipampa (Ferreñafe), Peru
Local name: Higuerón (Figure 2, left; Figure 3a)
Figure 2: Ficus americana: A. mature branch, B. leaf, C. terminal stipule, D. fruitful branch, E. mature syconium, F. bracts, G. ostiole. Ficus obtusifolia: H. mature branch, I. terminal stipule, J. immature syconia, K. mature syconia, L. cross-section of syconia, M. ostiole.
Figure 3: A. Ficus americana tree calculated to be more than 50 years old. B. In vitro seedlings of F. obtusifolia after six months of culture. C. Germplasm conservation of F. obtusifolia after 24 months of culture. D. Germplasm conservation of F. americana after 12 months of culture
General information: Hemiepiphytic or strangler tree, 10 to 30 m tall. Habitat: gallery forest, tropical evergreen forest, and tropical sub-deciduous forest, from 100 to 1500 m in elevation. Distribution: South America (Peru, Bolivia, Ecuador, Colombia, Brazil, Venezuela, Guyana), Greater Antilles, Lesser Antilles, Central America, and Mexico.
Morphological characteristics: External bark with lenticels and grooves; brown in color. Latex has a white to milky color and retains its color after 4 minutes of exposure. Terminal branches terete to elliptical, 0.3-0.5 cm in diameter. Terminal stipules 0.6-1.2 x 0.2-0.3 mm, glabrous. Petioles 0.5-3 cm x 1-2 mm, glabrous, ribbed. Leaf blade 3-10 x 1-4.5 cm, elliptical, margin entire, apex acute, base acute to cuneate, light green, glabrous and lustrous above, sparsely puberulous with whitish trichomes below, secondary veins in 10-18 pairs, anastomosed, tertiary cells forming polygonal cells along the veins. Peduncles 2.0-2.5 mm long and 0.5-1 mm in diameter. Syconium in pairs, opposite, axillary, globose, 0.3-0.5 cm in diameter, greenish when immature, blackish purple to reddish when fully ripe, glabrous. Bracts 3, entire or sometimes divided, elliptical, yellowish, persistent, inconspicuous, glabrous, brown when the syconia are mature, 1-2 x 2-3 mm. Ostiolus not prominent, circular, 1-2 mm diameter, with 3 occlusive bracts. Flowers whitish to yellowish when fresh.
Ficus obtusifolia Kunth, 1817
Local name: Higuerón (Figure 2, right)
Collection site: Pilasca (Salas, Lambayeque), Chiclayo (Chiclayo), and Kerguer (Lambayeque), Peru
General information: Hemiepiphytic or rupicolous tree, 12 to 35 m. Habitat: Lowland forest formation from 100 to 1650 m in elevation. Grows in both very wet areas and in seasonally very dry areas. Distribution: Bolivia, Brazil, Colombia, Belize, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Venezuela.
Morphological characteristics
External bark with lenticels and cracks, light brown. Latex milky white, oxidizes to pink when exposed. Terminal branches terete, 1 cm in diameter. Terminal stipules 10-40 x 4-15 mm, generally glabrous. Petioles 2-3 cm x 4-5 mm, glabrous, ribbed. Leaf-blade 8-26 x 3-11 cm, spatulate (obovate to oblanceolate), margin entire, obtuse at apex, cuneate to rounded at base, glabrous, glossy dark green on the upper surface with yellowish to dark brown veins, pale green on the underside, secondary veins in 5-10 pairs, anastomosed, tertiary veins forming polygonal cells, juvenile midrib cream-colored with a darker elliptical scar 2-4 mm long abaxially, merging close to the junction of the first base veins. Peduncles 2-3 mm long and 0.5 mm in diameter. Syconia in pairs, axillary, globose, 1-2 x 1-1.5 cm, greenish when immature, brown when mature, glabrous to puberulent. Surface spotted with dark brown to reddish lenticels, scattered to numerous, irregularly distributed. Bracts 2, whole or sometimes divided, deltoid, forming a greenish yellowish involucre when the syconia are immature, persistent, dark brown when the sycans mature, 7-15 x 6-8 mm, puberulent to glabrescent. Prominent ostiole, 3-5 mm in diameter, with 3 puberulent bracts. Flowers whitish to yellowish when fresh.
Seed contamination and germination
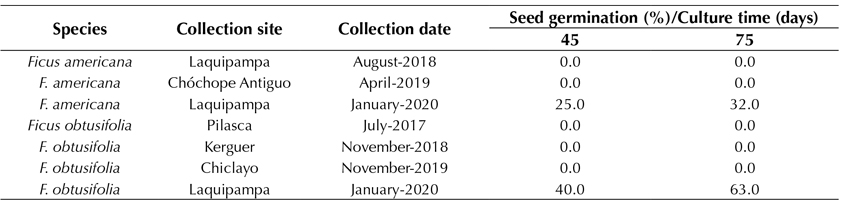
The seed contamination rate was 0%, which indicates that the disinfection protocol used on both the fruits and seeds was very efficient. Furthermore, the seed germination and seedling survival rates were between 95 and 100% for both species. This strongly indicates that not only did the disinfection protocol make it possible to obtain 100% aseptic seeds, but also that the germination rate and growth of the seedling maintained a very high success rate. After three months, following the collection of the seeds, the germination rate decreased to 90-100%. Leaving the seeds unsown for up to 12 months after being collected gradually decreased the germination rate to 32.0 and 63.3% in F. americana and F. obtusifolia, respectively (Table 1). Seeds older than one year and kept in dry sterile conditions from the moment of their collection did not germinate.
Table 1: In vitro seed germination of Ficus americana and F. obtusifolia, after 45 and 60 days of culture (culture date: February 2021)
Micropropagation and acclimatization
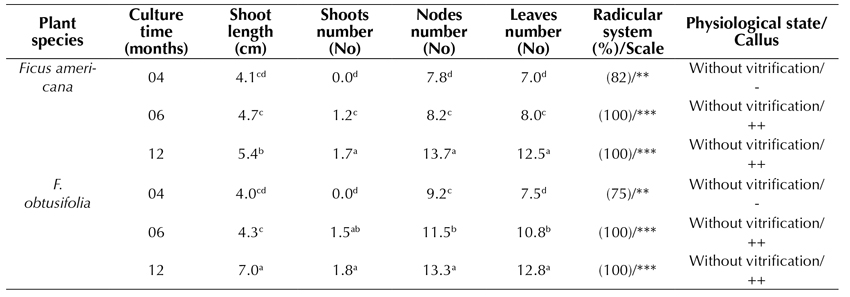
In the formulation of the Piper culture medium (MS supplemented with IAA 0.02 mg.L-1 and GA3 0.02 mg.L-1), the shoot elongation rate after 4 and 6 months of culture was similar in both species (Table 2, Figure 3b). However, after 12 months of culture, the highest elongation rate was reached in F. obtusifolia (7.0 cm). The number of shoots (1-2), nodes (12-13), and leaves (12) formed was similar in both species, increasing gradually with time in culture. Both species showed an optimal root system with more than 4 roots formed from 1 to 5 cm in length. The slight formation of a basal callus was also observed after 6 to 12 months following cultivation. Seedlings showed neither vitrification nor phenolization.
Piper medium: MS, 2.0% sacarose, vitamins, 0.02 IAA mg.L-1 and 0.02 mg.L-1 GA3
Test tubes: 150x25 mm -, without callus; +, < 1.0 cm Ø; ++; 1.0-2.0 cm Ø; +++, > 2.0 cm Ø -, without roots; *, 1-2 roots, **, 3-4 roots; ***, > 4 roots. Roots: 1-5 cm length Tukey parametric test at a significance level of 95%.
Table 2: Effect of Piper formulation in MS medium on in vitro propagated plants of Ficus americana and F. obtusifolia, after 4, 6, and 12 months of culture
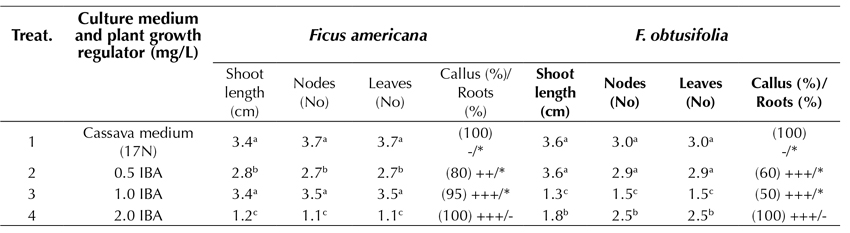
Optimal root development in in vitro plantlets is an important condition, which is sometimes fundamental for successful acclimatization and survival. By using 100 x 13 mm test tubes and studying the plantlets for 45 days of culture, the plants of F. americana reached the highest elongation of the shoot (3.4 cm) in the treatments that employed a cassava-based medium (17N) and 1.0 mg.L-1 IBA. Under the same growing conditions, but in a slightly more diluted medium of 0.5 mg.L-1 IBA, the F. obtusifolia plantlets achieved the highest elongation of the shoot (3.6 cm). It was therefore concluded that the cassava medium provided the best physiological response in the samples, which included a greater elongation of the shoot and no callus formation at the base of the explant or the roots. However, using a treatment with 2.0 mg.L-1 IBA, the over-contamination of the substrate lowered the root development efficiency, and the callus formation was 100%. In addition, there was an inadequate root formation (Table 3).
Cassava medium (17N): 1/3 MS, 2.0 sacarose, vitamins, 0.01 mg.L-1 NAA and 0.01 mg.L-1 GA3 Test tubes: 100x13 mm Ø; -, without callus; +, < 1.0 cm Ø; ++; 1.0-2.0 cm Ø; +++, > 2.0 cm Ø -, without roots; *, 1-2 roots, **, 3-4 roots; ***, > 4 roots. Roots: 1-5 cm length Tukey parametric test at a significance level of 95%.
Table 3: Effect of several formulations of plant growth regulators in MS medium on in vitro root induction in propagated plants of Ficus americana and F. obtusifolia after 45 days of culture
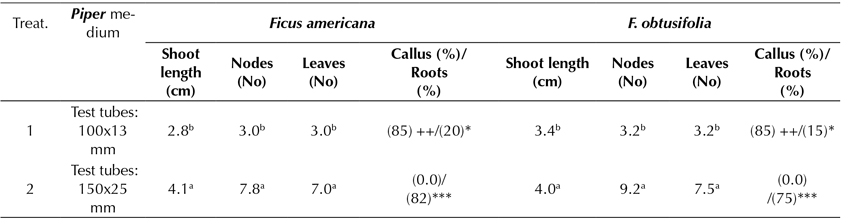
In an additional experiment, the effect of the glass container size used on the accessions was examined (test tubes of 100 x 13 mm vs. test tubes of 150 x 25 mm), showing significant differences while using the Piper culture medium as a proxy (Table 4). In both F. americana and F. obtusifolia, the highest elongation of shoots, nodes, and leaves was achieved when the specimen was grown in a glass container of 150 x 25 mm test tubes. The highest rooting was also improved by an average of 82 and 75%, respectively. No callus formation was observed in either specimen. However, when the glass container size decreased to 100 x 13 mm, an 85% callus formation was observed, and the rooting development was not greater than 20% in relative terms.
Piper medium: MS, 2.0 sacarose, vitamins, 0.02 IAA mg.L-1 and 0.02 mg.L-1 GA3 -, without callus; +, < 1.0 cm Ø; ++; 1.0-2.0 cm Ø; +++, > 2.0 cm Ø -, without roots; *, 1-2 roots, **, 3-4 roots; ***, > 4 roots. Roots: 1-5 cm length Tukey parametric test at a significance level of 95%.
Table 4: Effect of Piper medium on in vitro root induction in propagated plants of Ficus americana and F. obtusifolia, following 4 months of culture
Germplasm conservation
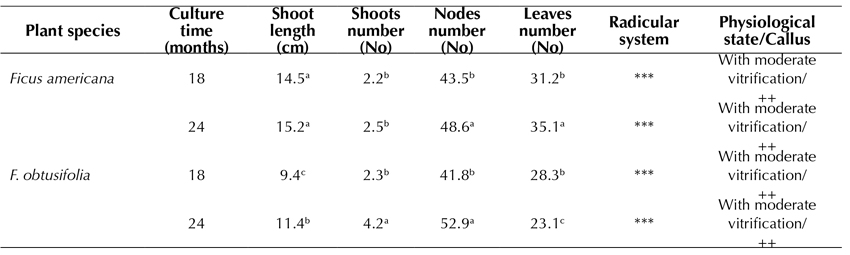
The germplasm conservation of both F. americana (Figure 3d) and F. obtusifolia (Figure 3c) was observed for up to 24 months during this study. F. americana reached the highest elongation of the shoot (15.2 cm) (Table 5), whereas, in F. obtusifolia, the highest number of nodes formed (52.9), thus allowing for a faster multiplication of explants for subcultures. In both species, the defoliation rate was quite high, and the vitrification and callus induction rates appeared to be moderate. The root system reached the highest development (Table 5).
Piper medium: MS, 2.0% sacarose, vitamins, 0.02 IAA mg.L-1 and 0.02 mg.L-1 GA3 Test tubes: 150x25 mm -, without callus; +, < 1.0 cm Ø; ++; 1.0-2.0 cm Ø; +++, > 2.0 cm Ø -, without roots; *, 1-2 roots, **, 3-4 roots; ***, > 4 roots. Roots: 1-5 cm length Tukey parametric test at a significance level of 95%.
Table 5: Effect of Piper medium in MS medium on in vitro germplasm conservation of Ficus americana and F. obtusifolia after 18 and 24 months of culture
Acclimatization
The acclimatization and survival rate in professionally sterilized farmland + vermiculite substrate (2: 1) was calculated as 64% for F. americana and 48% for F. obtusifolia while using relatively moderate data (Figures 4a, 4b, and 4c).
Figure 4: Acclimatization of transplanted plants under greenhouse conditions. A. Ficus americana (left) and F. obtusifolia (right) after two months of culture. B. Germplasm conservation of F. americana after 12 months of culture. F. americana and C. F. obtusifolia after 12 months of culture.
DISCUSSION
In the flora of Peru, F. americana has been reported in the high Andean and Amazonian regions of Huánuco, Junín, Loreto, Madre de Dios, Pasco, and San Martín, but it has not yet been reported in Lambayeque. F. obtusifolia has also been reported in the regions of Cuzco, Junín, and Loreto, but it has also not been reported in Lambayeque (Brako & Zarucchi, 1993). Therefore, this could be one of the first studies that analyze the taxonomy and propagation of these two species as part of the flora in the region of Lambayeque, on the northern coast of Peru. The morphological description of the species studied herein coincides with those reported by Cáceres and Reynel (2010) and Ibarra-Manríquez et al. (2012) in their works on the species of the genus Ficus in Chanchamayo (Junín, Peru) and Mexico.
In most studies on the micropropagation of the common fig (F. carica) carried out in regions where the species is native, shoot tips of plants collected directly from the field have been most commonly used as starting material (Bayoudh et al., 2015; Al-Shomali et al., 2017). However, a study carried out by Patah et al. (2018) employed ripe fruit and seeds, but the contamination and germination were not reported. Fertile seed production in figs is a complicated issue, given the need for the specific pollinator Blastophaga psenes L. to carry out the pollination, and, in some countries such as Brazil, the production of seedlings of F. carica is not possible due to the absence of B. psenes (Anjam et al., 2017). Therefore, other methods of propagation, such as burrs, plunging, grafting, tissue culture, and especially cuttings, are frequently used instead of pollination (Boliani et al., 2019). In other species such as F. religiosa, leaf explants are used for its multiplication (Siwach & Gill, 2014).
As for F. americana and F. obtusifolia, fresh syconium is consumed by various species of birds, rodents and small primates (‘keystone species’), which therefore constitute the main seed dispersers. The fertility of these seeds indicates the presence of their natural pollinators (Cáceres & Reynel, 2010), which are likely to be wasps of the Agaonidae family, specifically species of the genus Pegoscapus (Machado et al., 2005). This wasp species confirms the Ficus-insect coevolutionary interaction that began at the end of the Cretaceous (Datwyler & Weiblen, 2004) and resulted in both species being relatively abundant and out of conservation risk (LC/Least Concern) (Cáceres & Reynel, 2010). However, at the collection sites of these species in Lambayeque, only sporadic individuals were found due to the fact that the limits of natural regeneration are restricted to some streams that maintain little humidity throughout the year.
The Piper culture medium has been widely used in the micropropagation of numerous Piper species (Piperaceae) collected in both Peru and Brazil (Delgado-Paredes et al., 2012). It has been shown to reach high micropropagation rates in Piper tuberculatum, an important species with biocidal properties (Delgado-Paredes et al., 2017). In a similar way, it is used widely in the micropropagation of woody species collected from the Seasonally Dry Tropical Forest (SDTF) of northern Peru (Delgado-Paredes et al., 2020a), such as Muntingia calabura L. (Duque-Aurazo et al., 2020). The Piper medium has proven to stimulate root formation and induce shoot elongation in Piper species, and it could be used on its own without the need for two different formulations.
Conventionally, most efforts aimed at the micropropagation of Ficus have been carried out for the commercially important common fig (F. carica), which is grown as an important food crop worldwide (Singh et al., 2016). Other Ficus species, especially the Old-World species, are commonly propagated under in vitro conditions from explants (shoot tips) obtained from wild-collected plants. The highest number of propagations for commercially grown F. benjamina was obtained using an MS culture with 1.0 mg.L-1 BAP and 50.0 mg.L-1 adenine sulfate (Lavanya et al., 2006). For F. religiosa, the highest micropropagation rate was achieved using an MS culture medium supplemented with 5.0 mg.L-1 BAP via leaf explants (Siwach & Gill, 2014). So far, no studies on the micropropagation of the native American species are known to exist, and our study on F. americana and F. obtusifolia using a single culture medium (Piper) provides a foundation to this taxonomic window by showing the significant elongation and rooting of the sampled plants.
The cassava medium (17N) was formulated at the Biotechnology Research Unit of the International Center for Tropical Agriculture (CIAT, Cali, Colombia) and used in the rooting of more than 5000 cassava (Manihot esculenta Crantz) accessions (Roca, 1982). Furthermore, it has been used in the international transfer of cassava germplasm between countries and continents (Flores-Meza et al., 2014). This culture medium, whose formulation incorporates the MS macronutrients at a 1/3 concentration and only very low concentrations of growth regulators, does not induce callus formation. The traces of gibberellin (GA3) in the medium promote shoot elongation, and the traces of auxin (NAA) aid the development of an optimal root system.
The successful rooting of shoots obtained from plants collected in the field has been reported for several wild species of Ficus with varying trace compounds used in the culture medium. In F. benjamina, 1.0 mg.L-1 NAA and 1.0 mg.L-1 activated carbon were used (Lavanya et al., 2006), as well as 2.0 mg.L-1 IBA and 0.1 mg.L-1 IAA in F. religiosa (Siwach & Gill, 2014). In all the studies mentioned above, as well as in the one presented herein, the various concentrations of auxins IAA, IBA, and NAA have played a fundamental role in the rooting of the accessions.
Numerous previous studies have shown the effects of the container size, type, and quality induced on the propagation and conservation of the germplasm of different plant species. These studies generally agree that polycarbonate boxes are a better type of container for the storage of photosensitive Senecio macrophyllus M. Bieb. shoots, as opposed to glass tubes (Trejgell et al., 2015). Additionally, the micropropagation of peach rootstock GF 677 showed the best micro-plant development when grown in square vitro vent containers and glass jars (Kornova & Popov, 2009). It is possible that the smaller glass containers provide less airflow, which allows for a greater accumulation of ethylene, thus inhibiting shoot growth and root formation, instead of stimulating callus formation in the root.
In vitro tissue culture techniques are among the most important methods for multiplication in plant biotechnology. Among these techniques, one of the most important is the conservation of the germplasm under controlled conditions, especially for vegetatively propagated species (Delgado-Paredes et al., 2021). The asexual propagation of species by means of tissues is vital to reproducing vast quantities of plants that naturally produce low numbers of seeds, or species with seeds that quickly lose their viability and cannot be stored by reducing humidity or lowering temperatures (Engelmann, 1997; Reed et al., 2011; Rajasekharan & Sahijram, 2015). This largely includes the members of the genus Ficus and has proven to be a potentially costly loss of fertile material and a pronounced constraint to the large-scale production of its members. This is why the results obtained for F. americana and F. obtusifolia, which show that in vitro conservation can be extended for more than 24 months, open the possibility of conserving the best genotypes of these species. In addition, this can be subsequentially used in areas that are currently threatened by deforestation and other forms of land exploitation, which has been particularly observed in the seasonally dry tropical forest (SDTF) of Pilasca (Lambayeque, Peru) (Delgado-Paredes et al., 2020b).
In F. religiosa, the survival rate of seedlings obtained by direct organogenesis was 90% (Siwach & Gill, 2014), and that of those obtained by indirect organogenesis was 96.6% (Hesami et al., 2018). Said results are significantly higher than those reported in this present study.
CONCLUSION
Ficus americana and F. obtusifolia are evergreen species with high biomass production in the seasonally dry tropical forest of Lambayeque and northern Peru. This study developed an efficient protocol for micropropagation, rooting, acclimatization, and germplasm conservation using in vitro germinated seeds. Thus, it is now possible to develop an extensive reforestation program in currently deforested areas, contributing to the protection of the environment and the mitigation of climate change. This constitutes an important aspect of our university’s mission of social responsibility.
Acknowledgements
ACKNOWLEDGEMENTS
The authors are grateful to Nicole Mitidieri for their help in determining the species and consulting the descriptions, as well as to Alain Monsalve-Mera for improving the use of the English language in this document.
REFERENCES
Licencia
Derechos de autor 2023 Colombia forestal

Esta obra está bajo una licencia internacional Creative Commons Atribución-CompartirIgual 4.0.
Colombia Forestal conserva los derechos patrimoniales (copyright) de las obras publicadas, y favorece y permite la reutilización de las mismas bajo la licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional por lo cual se pueden copiar, usar, difundir, transmitir y exponer públicamente, siempre que:
Se reconozcan los créditos de la obra de la manera especificada por el autor o el licenciante (pero no de una manera que sugiera que tiene su apoyo o que apoyan el uso que hace de su obra).