DOI:
https://doi.org/10.14483/23448393.23475Published:
2025-12-09Issue:
Vol. 30 No. 3 (2025): September-DecemberSection:
Civil and Environmental EngineeringA Novel Rotational Limestone Treatment System for Effective Acid Mine Drainage Remediation
Un sistema novedoso de tratamiento rotacional de roca caliza para la remediación eficaz del drenaje ácido de minas
Keywords:
Coal mining, Acid mine drainage, rotational system, superficial degradation (en).Keywords:
Minería del carbón, drenaje ácido de mina, sistema rotacional, degradación superficial (es).Downloads
Abstract (en)
Context: The mining industry is the main culprit behind the generation of acid mine drainage (AMD). During coal extraction processes, sulfide minerals react with groundwater, releasing ions such as Fe2+ and Fe3+, sulfates (SO4-2), and protonic acidity (H+). The low pH of AMD can cause significant environmental damage. AMD remediation is usually achieved using alkaline systems, wherein the AMD passes through limestone to be neutralized. Nevertheless, this process requires prolonged treatment times and constant cleaning steps to remove the coating formed on the limestone, which reduces its effectiveness.
Method: This study evaluates a novel oxic-limestone rotational system for the treatment of AMD produced by the coal industry. The AMD collected was characterized in terms of pH, dissolved oxygen, Fe (Fe total, Fe2+, and Fe3+), and .
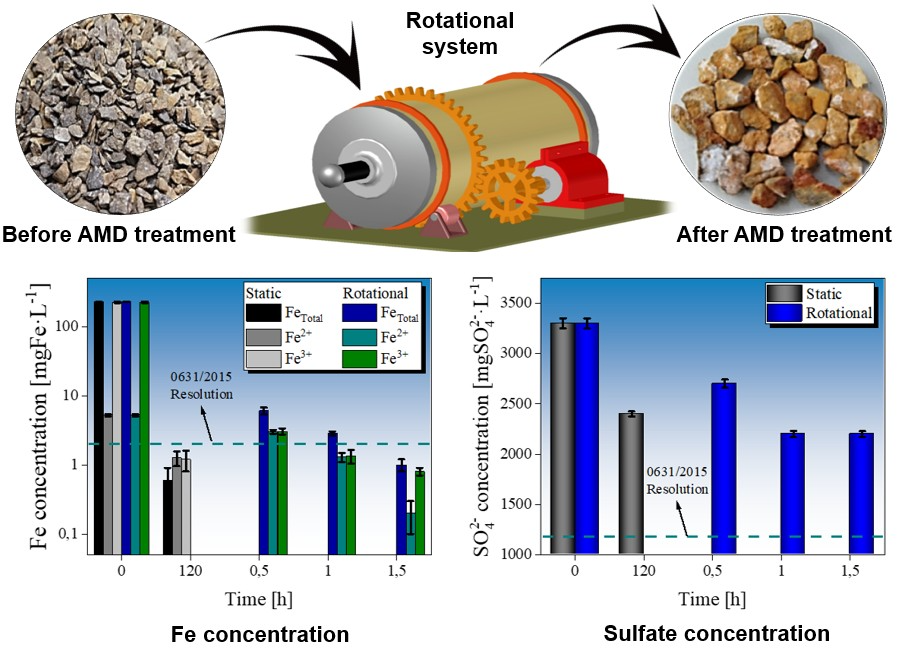
Results: The results demonstrate the optimal efficiency of the proposed system, reducing the treatment time from 120 h in conventional systems to 1.5 h when applying a ratio of 0.25k g of limestone per liter of AMD.
Conclusions: The rotational system enables the superficial degradation of the limestone, maintaining an active contact area for longer periods. This allows for optimized AMD remediation efficiency, reducing operating costs and necessitating fewer system cleanup steps.
Abstract (es)
Contexto: La industria minera es el principal responsable de la generación de drenaje ácido minero (DAM). Durante los procesos de extracción del carbón, los minerales sulfurados reaccionan con las aguas subterráneas, liberando iones como Fe2+ y Fe3+, sulfatos (SO4-2), y acidez protónica (H+). El bajo pH del DAM puede generar importantes daños medioambientales. La remediación del DAM generalmente se realiza a través de sistemas alcalinos, donde el DAM pasa a través de roca caliza para ser neutralizado. No obstante, este proceso requiere tiempos prolongados de tratamiento y etapas de limpieza constante para eliminar el recubrimiento formado en la roca, lo que reduce su eficacia.
Método: Este estudio evalúa un novedoso sistema rotacional de óxido-caliza para el tratamiento de DAM producido por la industria del carbón. El DAM recolectado se caracterizó en términos de pH, oxígeno disuelto, Fe (Fe total, Fe2+ y Fe3+), y SO4-2.
Resultados: Los resultados demuestran la eficiencia óptima del sistema propuesto, que reduce el tiempo de tratamiento de 120 h con sistemas convencionales a 1.5 h al aplicar una relación de 0.25 kg de caliza por litro de DAM.
Conclusiones: El sistema rotacional facilita la degradación superficial de la caliza, manteniendo un área de contacto activa durante periodos más largos. Esto permite optimizar la eficiencia de remediación del DAM, reduciendo los costes de operación y las etapas de limpieza requeridas por el sistema.
References
[1] G. Naidu, S. Ryu, R. Thiruvenkatachari, Y. Choi, S. Jeong, and S. Vigneswaran, “A critical review on remediation, reuse, and resource recovery from acid mine drainage,” Environ. Poll., vol. 247, pp. 1110–1124, Apr. 2019. https://doi.org/10.1016/j.envpol.2019.01.085
[2] H. E. Ben Ali, C. M. Neculita, J. W. Molson, A. Maqsoud, and G. J. Zagury, “Performance of passive systems for mine drainage treatment at low temperature and high salinity: A review,” Miner. Eng., vol. 134, pp. 325–344, Apr. 2019. https://doi.org/10.1016/j.mineng.2019.02.010
[3] D. Silva, C. Weber, and C. Oliveira, “Neutralization and uptake of pollutant cations from acid mine drainage (amd) using limestones and zeolites in a pilot-scale passive treatment system,” Miner. Eng., vol. 170, art. 107000, Aug. 2021. https://doi.org/10.1016/j.mineng.2021.107000
[4] J. S. Pozo-Antonio, I. Puente-Luna, S. L. López, and M. V. Ríos, “Tratamiento microbiano de aguas ácidas resultantes de la actividad minera: una revisión,” Tecno. Cien. Agua, vol. 8, no. 3, pp. 75–91, 2017. https://doi.org/10.24850/j-tyca-2017-03-05
[5] I. Park et al., “A review of recent strategies for acid mine drainage prevention and mine tailings recycling,” Chemosphere, vol. 219, pp. 588–606, Mar. 2019. https://doi.org/10.1016/j.chemosphere.2018.11.053
[6] I. Labastida, M. A. Armienta, R. H. Lara, R. Briones, I. González, and F. Romero, “Kinetic approach for the appropriate selection of indigenous limestones for acid mine drainage treatment with passive systems,” Sci. Total Environ., vol. 677, pp. 404–417, Aug. 2019. https://doi.org/10.1016/j.scitotenv.2019.04.373
[7] D. M. Acosta-Bueno, “Impactos ambientales de la minería de carbón y su relación con los problemas de salud de la población del municipio de Samacá (Boyacá), según reportes ASIS 2005-2011,” Specialization dissertation, Dept. Ed. Sci., Udistrital, Bogotá, Colombia, 2016.
[8] C. UPME, Ministerio de Minas y Energías, “Plan nacional de desarrollo minero con horizonte a 2025: Minería responsable con el territorio,” 2017. [Online]. Available: https://www1.upme.gov.co/simco/PlaneacionSector/Documents/PNDM_Dic2017.pdf
[9] R. H. Garzón, “Minería del carbón en Boyacá: entre la informalidad minera, la crisis de un sector y su potencial para el desarrollo,” Zero, vol. 33, no. 2344–8431, 2015. [Online]. Available: https://zero.uexternado.edu.co/mineria-del-carbon-en-boyaca-entre-la-informalidad-minera-la-crisis-de-un-sector-y-su-potencial-para-el-desarrollo/
[10] C. A. Agudelo Calderón, J. C. García-Ubaqie, R. Robledo Martínez, C. A. García-Ubaque, and L. Quiroz-Arcentales, “Evaluación de condiciones ambientales: aire, agua y suelos en áreas de actividad minera en Boyacá, Colombia,” Rev. Salud Púb., vol. 18, no. 1, pp. 50–60, Apr. 2016. https://doi.org/10.15446/rsap.v18n1.55384
[11] A. L. Boyles et al., “Systematic review of community health impacts of mountaintop removal mining,” Environ. Int., vol. 107, pp. 163–172, Oct. 2017. https://doi.org/10.1016/j.envint.2017.07.002
[12] I. Moodley, C. M. Sheridan, U. Kappelmeyer, and A. Akcil, “Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products,” Miner. Eng., vol. 126, pp. 207–220, Sep. 2018. https://doi.org/10.1016/j.mineng.2017.08.008
[13] L. E. Bertassello, P. S. C. Rao, J. Park, J. W. Jawitz, and G. Botter, “Stochastic modeling of wetland-groundwater systems,” Adv. Water Resour., vol. 112, pp. 214–223, Feb. 2018. https://doi.org/10.1016/j.advwatres.2017.12.007
[14] C. Zipper, J. Skousen, and C. Jage, “Passive treatment of acid-mine drainage,” Virgina Cooperative Extension, 2018. [Online]. Available: https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/460/460-133/CSES-216.pdf
[15] H. L. Yadav and A. Jamal, “Removal of heavy metals from acid mine drainage: A review,” Int. J. New Technol. Sci. Eng., vol. 2, no. 3, pp. 77–84, 2015. ISSN 2349-0780. Available: https://www.ijntse.com/upload/1443503976hlyand%20ajamal%20sir%202015.pdf
[16] D. Bejan and N. J. Bunce, “Acid mine drainage: electrochemical approaches to prevention and remediation of acidity and toxic metals,” J. Appl. Electrochem., vol. 45, no. 12, pp. 1239–1254, Dec. 2015. https://doi.org/10.1007/s10800-015-0884-2
[17] J. G. Skousen, P. F. Ziemkiewicz, and L. M. McDonald, “Acid mine drainage formation, control and treatment: Approaches and strategies,” Extr. Ind. Soc., vol. 6, no. 1, pp. 241–249, Jan. 2019. https://doi.org/10.1016/j.exis.2018.09.008
[18] G. R. Watzlaf, K. T. Schroeder, and C. L. Kairies, “Long-term performance of anoxic limestone drains,” Mine Water Environ., vol. 19, no. 2, pp. 98–110, Sep. 2000. https://doi.org/10.1007/BF02687258
[19] J. Skousen et al., “Review of passive systems for acid mine drainage treatment,” Mine Water Environ., vol. 36, no. 1, pp. 133–153, Mar. 2017. https://doi.org/10.1007/s10230-016-0417-1
[20] J. E. Santos Jallath, F. M. Romero, R. Iturbe Argüelles, A. Cervantes Macedo, and J. Goslinga Arenas, “Acid drainage neutralization and trace metals removal by a two-step system with carbonated rocks, Estado de Mexico, Mexico,” Environ. Earth Sci., vol. 77, no. 3, p. 86, Feb. 2018. https://doi.org/10.1007/s12665-018-7248-2
[21] C. R. Blanco-Zuñiga, N. Rojas-Arias, L. Y. Peña-Pardo, M. E. Mendoza-Oliveros, and S. A. Martinez-Ovalle, “Study of the influence of clays on the transfer of dissolved oxygen in water,” Ingeniería, vol. 26, no. 1, pp. 1–8, 2021. https://doi.org/10.14483/23448393.15846
[22] Y. Bao, C. Guo, G. Lu, X. Yi, H. Wang, and Z. Dang, “Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine,” Sci. Total Environ., vol. 616–617, pp. 647–657, Mar. 2018. https://doi.org/10.1016/j.scitotenv.2017.10.273
[23] E. W. Rice, R. B. Baird, and A. Eaton, Standard methods for the examination of water and wastewater, 23rd ed. Washington DC, USA: American Public Health Association, American Water Works Association, Water Environment Federation, 2017. https://yabesh.ir/wp-content/uploads/2018/02/Standard-Methods-23rd-Perv.pdf
[24] J. Skousen, A. Sexstone, K. Garbutt, and J. Sencindiver, “Wetlands for treating acid mine drainage,” Green Lands, vol. 22, no. 4, pp. 31–39, 1992.
[25] P. F. Ziemkiewicz, J. G. Skousen, D. L. Brant, P. L. Sterner, and R. J. Lovett, “Acid mine drainage treatment with armored limestone in open limestone channels,” J. Environ. Qual., vol. 26, no. 4, pp. 1017–1024, Jul. 1997. https://doi.org/10.2134/jeq1997.00472425002600040013x
[26] L. Merrill and W. A. Bassett, “The crystal structure of CaCO3 (II), a high-pressure metastable phase of calcium carbonate,” Acta Crystallogr. B, vol. 31, no. 2, pp. 343–349, Feb. 1975. https://doi.org/10.1107/S0567740875002774
[27] P. L. Althoff, “Structural refinements of dolomite and a magnesian calcite and implications for dolomite formation in the marine environment,” Amer. Miner. J. Earth Planet. Mat., vol. 62, pp. 772–783, 1977.
[28] K. Dong, H. Morikawa, S. Iwai, and H. Aoki, “The crystal structure of magnesite,” Amer. Miner. J. Earth Planet. Mat., vol. 58, no. 11, pp. 1029–1033, 1973.
[29] H. d’Amour, W. Denner, and H. Schulz, “Structure determination of α-quartz up to 68 x 10^8 Pa,” Acta Crystallogr. B, vol. 35, no. 3, pp. 550–555, Mar. 1979. https://doi.org/10.1107/S056774087900412X
[30] J. A. N. Carlos, B. Barrios, M. Lucia, M. Castro, and S. M. Arenas, “Prospectiva estratégica en los procesos de extracción y del beneficio de la roca caliza en el norte del Cesar, Colombia,” Rev. Agunkuya, vol. 4, pp. 1–14, 2018. https://digitk.areandina.edu.co/server/api/core/bitstreams/d98c24ee-bd50-45b6-a541-cd80904882ce/content
[31] G. R. Watzlaf, K. T. Schroeder, and C. L. Kairies, “Long-term performance of anoxic limestone drains,” Mine Water Environ., vol. 19, no. 2, pp. 98–110, Sep. 2000. https://doi.org/10.1007/BF02687258
[32] Ministerio de Ambiente y Desarrollo Sostenible, “Resolución 0631 De 2015. Diario Oficial No. 49.486 de 18 de abril de 2015,” 2015. [Online]. Available: https://www.minambiente.gov.co/wp-content/uploads/2021/11/resolucion-631-de-2015.pdf
[33] L. Bernier, M. Aubertin, A. M. Dagenais, B. Bussière, L. Bienvenu, and J. Cyr, “Limestone drain design criteria in AMD passive treatment: theory, practice and hydrogeochemistry monitoring at Lorraine Mine Site, Temiscamingue,” CIM Minespace 2001 Québec, 2001. [Online]. Available: https://www.researchgate.net/profile/Louis-Bernier-3/publication/265411811_Limestone_Drain_Design_Criteria_in_AMD_Passive_Treatment_Theory_Practice_and_Hydrogeochemistry_Monitoring_at_Lorraine_Mine_Site_Temiscamingue/links/5757fc9e08aef6cbe3626507/Limestone-Drain-Design-Criteria-in-AMD-Passive-Treatment-Theory-Practice-and-Hydrogeochemistry-Monitoring-at-Lorraine-Mine-Site-Temiscamingue.pdf
[34] L. N. Plummer, D. L. Parkhurst, and T. M. L. Wigley, “Critical review of the kinetics of calcite dissolution and precipitation,” in Chemical Modeling in Aqueous Systems, New York, NY, USA: ACM, 1979, ACM Symposium Series, vol. 93, pp. 537–573. https://doi.org/10.1021/bk-1979-0093.ch025
[35] W. A. M. Fernando, I. M. S. K. Ilankoon, T. H. Syed, and M. Yellishetty, “Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review,” Miner. Eng., vol. 117, pp. 74–90, Mar. 2018. https://doi.org/10.1016/j.mineng.2017.12.004
[36] O. Aduvire, Drenaje acido de mina: generación y tratamiento. Madrid, Spain: Instituto Geológico y Minero de España Dirección de Recursos Minerales y Geoambiente, 2006.
[37] T. V. Rakotonimaro, C. M. Neculita, B. Bussière, T. Genty, and G. J. Zagury, “Performance assessment of laboratory and field-scale multi-step passive treatment of iron-rich acid mine drainage for design improvement,” Environ. Sci. Poll. Res., vol. 25, no. 18, pp. 17575–17589, Jun. 2018. https://doi.org/10.1007/s11356-018-1820-x
[38] M. Jouini, T. V. Rakotonimaro, C. M. Neculita, T. Genty, and M. Benzaazoua, “Stability of metal-rich residues from laboratory multi-step treatment system for ferriferous acid mine drainage,” Environ. Sci. Poll. Res., vol. 26, no. 35, pp. 35588–35601, Dec. 2019. https://doi.org/10.1007/s11356-019-04608-1
[39] B. A. Wills, “Grinding Mills,” in Mineral Processing Technology, 4th ed. Amsterdam, Netherlands, Elsevier, International Series on Materials Science and Technology, 1988, ch. 7, pp. 253–308. https://doi.org/10.1016/B978-0-08-034937-4.50016-8
[40] D. K. Nordstrom, D. W. Blowes, and C. J. Ptacek, “Hydrogeochemistry and microbiology of mine drainage: An update,” App. Geochem., vol. 57, pp. 3–16, Jun. 2015. https://doi.org/10.1016/j.apgeochem.2015.02.008
[41] R. L. Pendleton and D. Nickerson, “Soil colors and special Munsell soil color charts,” Soil Sci., vol. 71, no. 1, pp. 35–44, 1951.
[42] E. Iakovleva, E. Mäkilä, J. Salonen, M. Sitarz, S. Wang, and M. Sillanpää, “Acid mine drainage (AMD) treatment: Neutralization and toxic elements removal with unmodified and modified limestone,” Ecol. Eng., vol. 81, pp. 30–40, Aug. 2015. https://doi.org/10.1016/j.ecoleng.2015.04.046
[43] Y. Chai et al., “Experimental study and application of dolomite aeration oxidation filter bed for the treatment of acid mine drainage,” Miner. Eng., vol. 157, art. 106560, Oct. 2020. https://doi.org/10.1016/j.mineng.2020.106560
[44] J. Demchak, T. Morrow, and J. Skousen, “Treatment of acid mine drainage by four vertical flow wetlands in Pennsylvania,” Geochem. Explor. Environ. Analysis, vol. 1, no. 1, pp. 71–80, Feb. 2001. https://doi.org/10.1144/geochem.1.1.71
[45] F. Scholz and H. Kahlert, “The calculation of the solubility of metal hydroxides, oxide-hydroxides, and oxides, and their visualisation in logarithmic diagrams,” ChemTexts, vol. 1, no. 1, p. 7, Mar. 2015. https://doi.org/10.1007/s40828-015-0006-0
[46] H. A. Peláez Morales, M. C. Prada Fonseca, G. Caicedo Pineda, C. X. Moreno Herrera, and M. A. Márquez Godoy, “Influence of the initial relation of Fe3+/Fe2+ in the process of biodesulfuration of a coal sample solution,” Rev. Int. Cont. Amb., vol. 29, no. 2, pp. 2111–217, 2013. ISSN 0188-4999. Available: https://www.scielo.org.mx/pdf/rica/v29n2/v29n2a7.pdf
[47] C. A. Cravotta and G. R. Watzlaf, “Design and performance of limestone drains to increase pH and remove metals from acidic mine drainage,” in Handbook of Groundwater Remediation using Permeable Reactive Barriers, Amsterdam, Netherlands: Elsevier, 2003, pp. 19–66. https://doi.org/10.1016/B978-012513563-4/50006-2
[48] E. Azzali et al., “Mineralogical and chemical variations of ochreous precipitates from acid sulphate waters (asw) at the Roşia Montană gold mine (Romania),” Environ. Earth Sci., vol. 72, no. 9, pp. 3567–3584, Nov. 2014. https://doi.org/10.1007/s12665-014-3264-z
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
License
Copyright (c) 2025 Cesar René Blanco Zúñiga, Laura Daniela Ulloa-Amador, Nicolás Rojas-Arias

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
From the edition of the V23N3 of year 2018 forward, the Creative Commons License "Attribution-Non-Commercial - No Derivative Works " is changed to the following:
Attribution - Non-Commercial - Share the same: this license allows others to distribute, remix, retouch, and create from your work in a non-commercial way, as long as they give you credit and license their new creations under the same conditions.




2.jpg)