DOI:
https://doi.org/10.14483/udistrital.jour.colomb.for.2009.1.a01Publicado:
01-01-2009Número:
Vol. 12 Núm. 1 (2009): Enero-DiciembreSección:
Artículos de investigación científica y tecnológicaDispersión de semillas de la palma útil (Astrocaryum chambira Burret) en tres bosques amazónicos con diferente grado de intervención humana
SEED DISPERSAL OF A USEFUL PALM (Astrocaryum chambira Burret) IN THREE AMAZONIAN FORESTS WITH DIFFERENT HUMAN INTERVENTION
Palabras clave:
Amazon forest, chambira palm, seed predation, insect seed predation, rodents. (en).Palabras clave:
bosque amazónico, depredación de semillas, depredación por insectos, palma de chambira, roedores. (es).Referencias
Beckman, N. G. & H. C. Muller-Landau. 2007. Differential effects of hunting on pre-dispersal seed predation and primary and secondary seed removal of two neotropical tree species. Biotropica 39: 328-339.
Bodmer, R. E., R. Aquino, P. E. Puertas, C. J. Reyes, T. G. Fang & N. L. Gottdenker. 1997. Manejo y uso sustentable de pecaríes en la Amazonía peruana. Comisión de Supervivencia de Especies. Lima, Perú. Pg. 102.
Borgoft, P. 1994. Mocora palm-fibers: Use and management of Astrocaryum standleyanum (Arecaceae) in Ecuador. Economic Botany 48: 310-325.
Burret, M. 1934. Die palmengattung Astrocaryum GFW Myer Repertorium Specierum. Novarum Regni Vegetabilis 35: 114-158.
Bustamante, R. O. & J. A. Simonetti. 2000. Seed predation and seedling recruitment in plants: the effect of the distance between parents. Plant Ecology 147: 173-183.
Calderón, E., G. Galeano & N. García. 2005. Libro rojo de plantas de Colombia. Volumen 2: Palmas, frailejones y zamias. Serie Libros Rojos de Especies Amenazadas de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Instituto de Ciencias Naturales Universidad Nacional de Colombia, Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Bogotá.
Carrillo, E., G. Wong & A. D. Cuarón. 2000. Monitoring mammal populations in Costa Rican Protected Areas under different hunting restrictions. Conservation Biology 14: 1580-1591.
Coomes, O. T. 2004. Rain forest 'conservationthrough-use'? Chambira palm fibre extraction and handicraft production in a land-constrained community, Peruvian Amazon. Biodiversity and Conservation 13: 351-360.
Cordeiro, N. J. & H. Howe. 2001. Low recruitment of trees dispersed by animals in African forest fragments. Conservation Biology 15 (6): 1733-1741.
Cox, D. R. 1972. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 34:187-220.
De La Hoz, N. 2007. Uso de fauna silvestre por las comunidades indígenas. En: Ruiz S. L., E. Sánchez, E. Tabares, A. Prieto, J. C. Arias, R. Gómez, D. Castellanos, P. García, L. Rodríguez (eds). Diversidad biológica y cultural del sur de la Amazonia colombiana - Diagnóstico. Corpoamazonia, Instituto Humboldt, Instituto Sinchi, uaespnn. Bogotá. Pp. 356-358.
Defler, T. R. 1996. Aspects of the ranging patterns in a group of wild woolly monkeys (Lagothrix lagotrhicha). American Journal of Primatology 38: 289-302.
Forget, P. M. 1990. Seed-dispersal of Vouacapoua americana (Caesalpiniaceae) by Caviomorph Rodents in French Guiana. Journal of Tropical Ecology 6: 459-468.
Forget, P. M. 1996. Removal of seeds of Carapa procera (Meliaceae) by rodents and their fate in rainforest in French Guiana. Journal of Tropical Ecology 12: 751-761.
Forget, P. M. & P. A. Jansen. 2007. Hunting increases dispersal limitation in the tree Carapa procera, a non-timber forest product. Conservation Biology 21: 106-113.
Forget, P. M. & T. Milleron. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87: 596-599.Giles, R. 1974. Wildlife management techniques. The Wildlife Society. Washington D.C. Pg. 633.
Haugaasen, T. & C. A. Peres. 2005. Mammal assemblage structure in Amazonian flooded and unflooded forests. Journal of Tropical Ecology 21: 133-145.
Henderson, A., Galeano, G. R. & R. Bernal. 1995. Field guide to the palms of the Americas. Princeton University Press. Princeton, New Jersey.
Henderson, A. 1997. The palms of the Amazons. Oxford University Press. Oxford, UK.
Jansen, P. A. 2003. Scatterhoarding and tree regeneration. Ecology of nut dispersal in a neotropical rainforest. Ph. D. thesis. Wageningen University. Wageningen.
Jansen, P. A. & P. M. Forget. 2001. Scatterhoarding and tree regeneration. En: Bongers, F., P. Charles-Dominique & P. M. Forget (eds.). Nouragues: dynamics and plant-animal interactions in a neotropical rainforest. Kluwer Academic Publishers. Ámsterdam. Pp. 275-288.
Janzen, D. H. 1971. Seed predation by animals. Annual Review of Ecology and Systematics 2: 465-492.
Janzen, D. H. 1972. Association of a rainforest palm and seed-eating beetles in Puerto Rico. Ecology 53: 258-261.
Jensen, O. H. & H. Balslev. 1995. Ethnobotany of the fiber palm Astrocaryum chambira (Arecaceae) in Amazonian Ecuador. Economic Botany: 309-319.
Jordano, P. & E. W. Schupp. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecological Monographs 70: 591-615.
Kahn, F. & F. Moussa. 1994. Diversity and conservation status of Peruvian palms. Biodiversity and Conservation 3: 227-241.
Kleinbaum, D. G. 1996. Removal analysis: a selflearning text. Springer. Nueva York.
Male, L. H. & T. V. Smulders. 2007. Hyperdispersed cache distributions reduce pilferage: a field study. Animal Behaviour 73: 717-726.
Nathan, R. & H. C. Muller-Landau. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology & Evolution 15: 278-285.
Palacios, E. A. & A. Rodríguez. 1995. Caracterización de la dieta y comportamiento alimentario de Callicebus torquatus lugens. Facultad de Ciencias, Departamento de Biología, Universidad Nacional de Colombia. Bogotá.
Peres, C. A. & E. Palacios. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal mediated seed dispersal. Biotropica 39: 305-315.
Pinilla, M. C. 2004. Uso del paisaje en el sector sur del Parque Natural Nacional Amacayacu (Amazonas, Colombia). Cuadernos de Desarrollo Rural 53: 133-156.
Pollock, K. H., S. R. Winterstein, C. M. Bunck & P. D. Curtis. 1989. Removal analysis in telemetry studies: The staggered entry design. The Journal of Wildlife Management 53: 7-15.
Rudas, A. & A. Prieto. 2004. Flórula del Parque Nacional Natural Amacayacu. Missouri Botanical Garden Press. San Luis, Missouri.
Sallabanks, R. & S. P. Courtney. 1992. Frugivory, seed predation, and insect-vertebrate interactions. Annual Review of Entomology 37: 377-400.
Schultes, R. E. 1977. Promising structural fiber palms of the Colombian Amazon. Principes 21: 72-82.
Smythe, N. 1989. Seed removal in the palm Astrocaryum standleyanum: Evidence for dependence upon its seed dispersers. Biotropica 21: 50-56.
SPSS v.14. 2005. SPSS. Chicago.
Silvius, K. M. & J. M. Fragoso. 2003. Redrumped agouti (Dasyprocta leporina) home range use in an Amazonian forest: implications for the aggregated distribution of forest trees. Biotropica 35: 74-83.
Stevenson, P. R., M. J. Quiñones & M. C. Castellanos. 2000a. Guía de frutos de los bosques del río Duda, Macarena, Colombia. Asociación para la Defensa de La Macarena - IUCN. Bogotá.
Stevenson, P. R., M. J. Quiñones & J. A. Ahumada. 2000b. Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica 32: 533-544.
Terborgh, J., G. Núñez-Iturri, N. C. A. Pitman, F. H. Cornejo-Valverde, P. Álvarez, V. Swamy, E. G. Pringle & C. E. T. Paine. 2008. Tree recruitment in an empty forest. Ecology 89: 1757-1768.
UAESPNN. 1999. Diagnóstico y estrategias de conservación de las poblaciones de fauna silvestre con mayor presión de caza en el sector sur del Parque Nacional Natural Amacayacu. Informe final. Parque Nacional Natural Amacayacu, U. R. A. O. y. P. (ed.). Ministerio de Ambiente, Vivienda y Desarrollo Territorial.
Vormisto, J. 2002. Making and marketing chambira hammocks and bags in the village of Brillo Nuevo, Northeastern Peru. Economic Botany 56: 27-40.
Xiao, Z., P. A. Jansen & Z. Zhang. 2006a. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. Forest Ecology and Management 223: 18-23.
Xiao, Z., Y. Wang, M. Harris & Z. Zhang. 2006b. Spatial and temporal variation of seed predation and removal of sympatric large-seeded species in relation to innate seed traits in a subtropical forest, Southwest China. Forest Ecology and Management 222: 46-54.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
SEED DISPERSAL OF A USEFUL PALM (Astrocaryum chambira Burret) IN THREE AMAZONIAN FORESTS WITH DIFFERENT HUMAN INTERVENTION
Dispersión de semillas de la palma útil (Astrocaryum chambira Burret) en tres bosques amazónicos con diferente grado de intervención humana
Dispersão de sementes da palma útil (Astrocaryum chambira Burret) nos três bosques amazônicos com diferentes graus da intervenção humana
Beatriz H. Ramírez1,2, Ángela Parrado-Rosselli3 & Pablo Stevenson1
1Laboratorio de Ecología de Bosques Tropicales y Primatología,
Centro de Investigaciones Ecológicas La Macarena, Departamento de Ciencias Biológicas
, Universidad de los Andes, AA. 4976, Bogotá,
pstevens@uniandes.edu.co
2Institute of Biodiversity and Ecosystem Dynamics (IBED), Universiteit van Amsterdam.
3Grupo Uso y Conservación de la Diversidad Forestal, Proyecto Curricular de
Ingeniería Forestal, Facultad del Medio Ambiente y Recursos Naturales, Universidad
Distrital Francisco José de Caldas. Correspondencia:
aparrador@udistrital.edu.co
Recepción: Mayo 27 de 2009/Aprobación: Septiembre 5 de 2009
ABSTRACT
The young leaves of Astrocaryum chambira are used by the indigenous people in the Amazon as raw material for handicrafts. However, few studies have been made on the natural history of this palm and on the indirect impact caused by the decrease of its dispersal agents. Considering that the loss of animal dispersal vectors due to hunting and landscape modification can affect seed dispersal proces ses of tropical forest plants, the goal of this study was to compare seed dispersal of A. chambira in three terra firme forests of the Colombian Amazon, with different degrees of human intervention. We censused densities of dispersal agents of A. chambira, and characterized the seed shadow. We also marked seeds to estimate dispersal distances, and established density and distance-dependent experimental stations to assess their relevance on seed dispersal. The results showed that seed removal was proportional to dispersal agent densities and forest intervention levels. Insects were the main seed predators in all sites but their effect was less pronounced in the low intervened forest site. Seed density did not show any effect on removal, while a higher probability of survival at intermediate distances from the parent palm (10 m) was found. Future studies should focus on seedling establishment, recruitment rates and the effects of human intervention on subsequent life stages of the palm.
Key words: Amazon forest, chambira palm, seed predation, insect seed predation, rodents.
RESUMEN
Las hojas jóvenes de Astrocaryum chambira son utilizadas por las comunidades indígenas amazónicas como materia prima para la fabricación de artesanías. Sin embargo, son muy pocos los estudios acerca de su historia de vida y de los impactos indirectos causados por la disminución de sus agentes dispersores. Teniendo en cuenta que la pérdida de animales dispersores de semillas por factores como cacería y modificación de hábitat afecta la dispersión de semillas de las especies de plantas tropicales, el objetivo de este estudio fue comparar la dispersión de semillas de A. chambira en tres bosques de tierra firme del Amazonas colombiano sujetos a diferentes niveles de intervención antrópica. Censamos las densidades de los agentes dispersores de A. chambira y caracterizamos la sombra de semillas. También marcamos semillas con el fin de estimar las distancias de dispersión y establecimos estaciones experimentales de densodistancio-dependencia para evaluar su relevancia en la dispersión de semillas de esta especie. Los resultados muestran que la remoción de semillas fue proporcional a la densidad de animales y al nivel de intervención del bosque. Los insectos fueron los principales depredadores en todos los sitios pero su efecto fue menos pronunciado en el bosque me-nos intervenido. La densidad de semillas no generó ningún efecto en la remoción, mientras que encontramos una mayor probabilidad de supervivencia a distancias intermedias de la palma (10 m). Estudios futuros se deberían enfocar en el establecimiento de las plántulas, las tasas de reclutamiento y el efecto de la intervención antrópica en los posteriores estadios de vida de esta palma tropical.
Palabras clave: bosque amazónico, depredación de semillas, depredación por insectos, palma de chambira, roedores.
RESUMO
As folhas jóvens de Astrocaryum chambira são utilizadas pelas comunidades indígenas amazônicas como matéria prima para a fabricação de artesanato. Sem embargo, são muito poucos os estudos acerca da sua historia de vida e dos impactos indiretos causados pela diminuição dos seus agentes dispersores. Tendo em conta que a perda de animais dispersores de sementes por fatores como a caça e a modificação de hábitat afeta a dispersão de sementes das espécies de plantas tropicais, o objetivo deste estudo foi comparar a dispersão de sementes de A. chambira em três bosques de terra firme do Amazonas colombiano sujeitos a diferentes níveis de intervenção antrópica. Censamos as densidades dos agentes dispersores de A. chambira e caracterizamos a sombra de sementes. Também marcamos sementes com a finalidade de estimar as distâncias de dispersão e estabelecer estações experimentais de denso distância-dependência para avaliar sua relevância na dispersão de sementes desta espécie. Os resultados mostram que a remoção de sementes foi proporcional a densidade dos animais e ao nível de intervenção do bosque. Os insetos foram os principais depredadores em todos os lugares, mas seu efeito foi menos pronunciado nos bosque não intervenidos. A densidade de sementes não gerou nenhum efeito na remoção, enquanto que encontramos una maior probabilidade de sobrevivência às distâncias intermediárias da palma (10 m). Estudos futuros deveríam se enfocar no estabelecimiento das mudas, as taxas de recrutamento e o efeito da intervenção antrópica nos posteriores estágios de vida desta palma tropical.
Palavras chave: Floresta Amazônica, depredação das sementes, depredação pelos insetos, palma de chambira, roedores.
INTRODUCTION
The chambira palm, Astrocaryum chambira Burret (1934) (Arecaceae), has been traditionally used by different indigenous communities of Amazonia (Schultes 1977, Jensen & Balslev 1995, Vormisto 2002). They obtain fibers from new leaves to produce nets, hammocks, household and ritual artifacts (Borgoft 1994, Jensen & Balslev 1995, Vormisto 2002). These products also generate economical income due to the interest of tourists in local handi-crafts (Jensen & Balslev 1995). The traditional harvest system consists on the removal of the youngest unfolded leaf, but leaving the next one intact in order to guarantee palm growth (Borgoft 1994, Jensen & Balslev 1995). However, it has been reported that several communities are not using this system anymore, harvesting the whole plant, and therefore affecting the development and growth regeneration dynamics of the species (Coomes 2004). Additionally to these direct stresses, other anthropogenic disturbances can alter the demography of the species. For example, the loss of animal dispersal vectors due to hunting and landscape modification can affect seed dispersal processes of tropical forest plants (Beckman & Muller-Landau 2007, Peres & Palacios 2007), which in turn have an effect on recruitment rates, genetic flux, coloni zation abilities and patterns of spatial distribution (Nathan & Muller-Landau 2000). Since trade of handicrafts has been increasing, but the exploitation depends exclusively on the natural population of the palm (Coomes 2004), it is unknown whether the natural populations of A. chambira will be able to tolerate such demand.
A. chambira is a palm species dispersed mainly by caviomorph rodents such as species of the genera Agouti, Dasyprocta and Myoprocta. These animals generally act as seed predators as they bury seeds to retrieve and eat them in periods of food scarcity. The successful seed dispersal occurs when the rodents fail to recover the buried seeds (Smythe 1989, Jansen & Forget 2001). The advantage of seed burial is that it reduces seed predation, as insects predate upon unburied seeds, particularly those that are not taken away from the parent palm (Janzen 1971, Forget 1990, Forget & Milleron 1991). The genera Agouti and Dasyprocta, commonly known as “borugos” and “guaras”, respectively, have been highly targeted by hunters. Additionally, although in several places of the Amazon Myoprocta is usually ignored (Carrillo et al. 2000, Peres & Palacios 2007), in the Colombian Amazonia they are highly hunted, particularly in intervened forests where they are found by dogs or caught with small traps by any member of an indigenous community working in the community garden (De la Hoz 2007). Thus, human intervention in the forest, generated by both planned and/or sporadic hunting, and proximity to settlements might be related with low animal population densities and affect regeneration dynamics of the species. For instance, Peres & Palacios (2007) have found lower population densities of caviomorph rodents in heavily hunted forests than in lightly hunted forests or non-hunted forests. Also, changes in mammal densities have shown differential effects on seed dispersal and seed predation depending on the traits and dispersal modes of the plant species (Bustamante & Simonetti 2000, Cordeiro & Howe 2001, Beckman & Muller-Landau 2007). In that way, knowledge of the seed dispersal processes of A. chambira under different intervention conditions might be very important for management purposes of its natural populations. Since A. chambira is one-large-seeded species that depends on secondary seed dispersal, a reduction in seed-disperser populations by hunting or any other form of human disturbance might re duce the proportion of removed seeds, and many of them will remain under the parent plant where the risk of mortality by seed predation is the highest (Forget & Jansen 2007).
In order to contribute to a better understanding of the consequences of changes in disperser populations for regeneration of tropical forest plants, we studied the seed fate of A. chambira in three forests subjected to different degree of human intervention, where densities of seed dispersers were supposed to be different. For this purpose, in each forest site we censused the dispersal agents, characterized the seed shadow and evaluated seed predation, seed removal by animals and seed dispersal distances. With lower disperser densities as a consequence of human intervention, we expected less seeds removed and shorter dispersal distances from the parent palm.
METHODS
STUDY AREA
Three Amazonian Tropical Humid Forest sites were selected for this study. The first two sites were located in Macedonia, an indigenous community on the northern margin of the Amazon River, state of Amazonas, Colombia (70° 13’ 22.4” W, 3° 49’ 01.0” S). Mean annual temperature is 26ºC and mean annual precipitation reaches values of 3200 mm, averaging 270 mm per month (Rudas & Prieto 2004). July and October are the driest months while January and April are the wettest months (UAESPNN 1999). The two sites were selected on the basis of the degree of human intervention according to Pinilla (2004) from high to moderate. The highly intervened forest (HIF) was considered as the forest continuously used by indigenous people for the extraction of both timber and non timber products (Pinilla 2004), including A. chambira leaves for craft-making purposes. This forest was located less than 5 km away from the community (modi fied from Pinilla 2004), where local people have used slash-and-burn cultivation systems, which have turned the forest into a mosaic of different regeneration stages. The moderately intervened forest (MIF ) consisted on forests regularly used for hunting and natural resources harvesting (planned journeys), including the extraction of A. chambira leaves, neither historic nor current cultivation. For the purposes of this study, this site was located more than 5 km away from the Macedonia settle ment (modified from Pinilla 2004).
The third forest was located at the biological station Mosiro Itajura located in the state of Vaupés, Colombia (69°31’2.9’’W, 1°04’21.8’’S). Mean annual temperature is 25.1° C and mean annual precipitation is between 3000 and 4000 mm (De fler 1996). May is the month with the highest precipitation (384 mm) and September is the driest month (258 mm: Palacios & Rodríguez 1995). The nearest indigenous community is located more than 8 km away in a straight line or more than 5 hours by foot. According to the local indigenous communities this is a sacred site, therefore hunting has always been restricted. Also, the existence since the 1980s of the biological station has entailed additional protection. There is no exploitation of young leaves of A. chambira or harvest of any other forest products. This forest was defined as the low intervened forest (LIF).
STUDY SPECIES
Astrocaryum chambira is a palm with a solitary erect trunk up to 22 m tall and 19-35 cm in dia meter (Henderson et al. 1995). The internodes are covered with black or grey spines of up to 20 cm in length. The leaf rachis is covered densely by yellow or brown flat spines of 3 up to 15 cm in length. Four to six leaves can be produced per year (Coomes 2004). Inflorescences are interfoliary and erect at anthesis, and in fruit. Fruits are obovoid of 5-6 cm in length by 4-4.5 cm in diameter, with yellow-green epicarp and with tiny spinules of white-brown color. The mesocarp is fibrous and yellow when ripe (Stevenson et al. 2000a). The endocarp is black, thick and bony with three lateral pores (Henderson et al. 1995). Each fruit is single-seeded and according to Bodmer et al. (1997) both fruits and seeds of the genus Astrocaryum are lipid-rich. Germination of the seeds occurs 8-10 months after they fall to the ground (Coomes 2004).
A. chambira is restricted to the Amazon basin and it can be found in terra firme , open vegetation and temporarily flooded forests (Várzea). It can be highly frequent at elevations below 350 m, and it is not categorized as an endangered species (Kahn & Moussa 1994, Henderson 1997, Calderón et al. 2005). A. chambira is distributed along the Amazon region of Colombia (Amazonas, Caque tá, Guaviare, Meta, Putumayo and Vaupés), Venezuela (Amazonas), Ecuador (Morona-Santiago, Napo), Perú (Amazonas, Loreto) and Brazil (Acre, Amazonas) (Henderson et al. 1995).
DATA COLLECTION
Data were collected from January to May 2004 at the HIF and the MIF, and from February to June 2005 at the LIF. In each forest we estimated density of seed dispersal agents, and chose eight fruiting individuals of A. chambira to characterize the seed shadow, seed removal and dispersal distances. The minimum distance between individuals was 100 m.
Census of seed dispersal agents
In each forest type, we estimated the density of dispersal agents of A. chambira, Dasyprocta fuliginosa (common name guara) and Myoprocta sp. (Myoprocta acouchy - Myoprocta exilis; common name tintín) by visual censuses on linear transects. The total length covered by the census was 60.6 km at the HIF, 56.7 km at the MIF and 129.4 km at the LIF. The effective transect width was determined by King’s method (Giles 1974). Although forests have been subjected to a different disturbance regime, altering both plant and animal species composition, there were not significant more open conditions at the HIF, as well as we did not sample in gardens or barbechs. Additionally, effective transect width was estimated based on the perpendicular distance from the centre of the survey path separately in each forest site, where the largest width was obtained at the LIFsite (mean 12.04 m). Thus, animal censuses were not biased in favor of a particular site. The average speed of the census was 2 km/h (SD = 0.3 km/h). Censuses were carried out over 3 months at least once per week, between 6:00 h and 10:00 h. Each time a D. fuliginosa or Myoprocta sp. was observed, the perpendicular distance to the transect and the number of individuals were recorded. The Kruskall-Wallis analysis was used in order to compare densities of each rodent species between forest types. Also, in order to define the differences between pair of forests Mann-Whitney U-tests were performed.
Seed shadow
Four of the eight adult individuals of A. chambira selected per forest were used to characterize the seed shadow created after the fruits fell from the palm. Around the trunk of each individual we established a 78.5 m2 area (5 m radius). From this 5 m radius we set six transects of 1 m × 50 m every 60°. Every fifteen days, we counted and classified all A. chambira seeds found within the 78.5 m 2 area and in each transect. Seeds were classified according to the seed’s condition: intact, predated by mam mals, or predated by insects. A seed was classified as intact when its endocarp was not perforated or broken. Evidence to classify a seed as predated by mammals consisted of broken seeds or pieces of endocarp found on the ground. When a seed presented holes that passed through the endocarp and reached the endosperm it was classified as predated by insects. In this case, these were classified into subgroups depending on the infesting agent. Samples of the predating insects were identified at the entomological collection of the Amsterdam Zoological Museum.
One-way analysis of variance (ANOVA) was performed to compare the differences between forests in the amount of fruits found in the 5 m radius area around each palm. Change in the percentage of predated seeds along time was obtained for forest site. We compared the proportion of predated seeds per forest at the 45th day after they began to fall to the ground. If there were significant differences we carried out a post hoc Tukey HSD test. All analyses were performed using the software SPSS version 14 (SPSS 2005).
Seed removal and dispersal distance
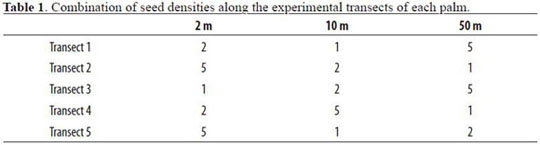
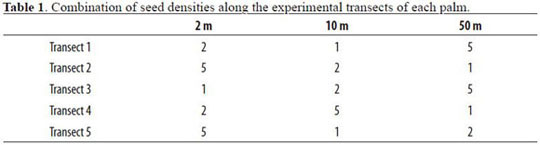
The other four individuals of A. chambira per forest site were used to experimentally assess seed removal by animals, dispersal distance and densi-transect had a different combination of distance ty and distance-dependent effects. In each palm, and density treatments (Table 1). On a biweekly every 72° we installed five 50 m transects from the basis, we counted the number of seeds per station trunk. In each transect we placed three stations at and determined their condition (intact, predated or 2, 10 and 50 m from the palm. On each station, missing). We never replaced predated or missing densities were of one, two and five seeds. Each seeds.
The Cox proportional hazard analysis (Cox 1972) was used to determine if the forest type, the distance from the parent palm or the seed density significantly affected potential seed survival. It was calculated as those seeds that remained intact in the station and/or those that were missing (assuming that removal enhances seed survival) minus those seeds predated. All variables were treated as cate gorical values. If differences were significant we showed the Hazard Ratio (HR), which indicates the increase in the probability of a seed to potentially survive when subjected to a particular treatment in comparison to the other one. To test for the proportional hazard assumption we visually examined the Log-Log plots for parallel curves (Kleinbaum 1996). We also estimated the mean removal time of the seeds at each forest type with a Kaplan-Meier removal analysis (Pollock et al. 1989). Analyses were made using SPSS version 14 (SPSS 2005).
For determining dispersal distances we marked 50 seeds per palm, all of them with green epicarp and yellow mesocarp. We marked each seed by attaching 50 cm of nylon line with 10 cm of vinyl fluo rescent tape (sensu Jansen 2003). To tie the seed, we drilled a hole through the tip of the seed without entering the endosperm and passed the nylon line through it. Due to the lack of electricity at the LIF forest, we used glue to attach the nylon thread to the seed. Seeds were placed in ten groups of five seeds at 3 m distance from the parent trunk and checked them every 15 days. If a seed was missing we looked for it along ten transects 50 m long that began from the trunk of the palm every 36°. For each seed found we measured the dispersal distance and depth. We also verified whether removed seeds found on previous revisions were still buried, and their condition was recorded only until the end of this study. Non-recovered seeds were classified as lost.
RESULTS
CENSUSES OF DISPERSAL AGENTS
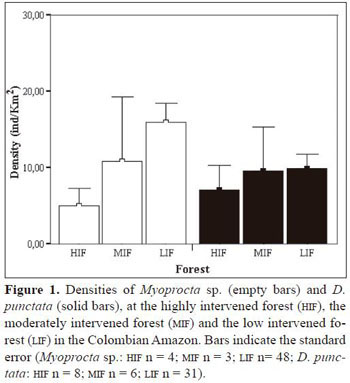
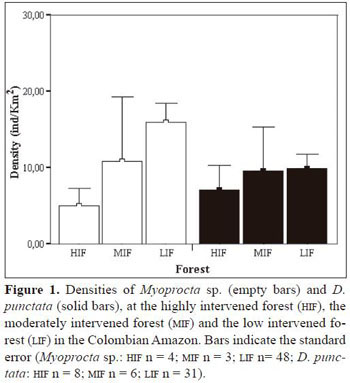
Mean density of D. fuliginosa individuals at the HIFwas 7 ind/km2, and 10 ind/km 2 at the MIF and at the LIF. Mean density of Myoprocta sp. at the HIF was 5 ind/km2, 11 ind/km2at the MIF and 16 ind/km2 at the LIF. Total D. fuliginosa and Myoprocta sp. sample size were 45 and 54 individuals in all forest sites, respectively. Although the number of individuals observed was small, the analysis showed significant differences in densities of Myoprocta sp. and D. fuliginosa among forests (D. fuliginosa: χ−22 = 9.4, P = 0.009, Myoprocta sp.:χ−22= 16.3, P < 0.001; Figure 1). Differences between the HIF and the MIF were not significant for both rodent species ( D. fuliginosa: Z = -0.3, P = 0.762, Myoprocta sp: Z = -0.5, P = 0.650). In contrast, we found significant differences between the LIF and the MIF for both D. fuliginosa and Myoprocta sp. (Z = -2.2, P = 0.029 and Z = -3.4, P = 0.001, respectively), and between the LIFand the HIF as well (D. fuliginosa: Z = -2.2, P = 0.008, Myoprocta sp: Z = -3.0, P = 0.003).
SEED SHADOWS AND SEED PREDATION
Our results showed that all seeds produced by A. chambira palms in the three forest sites fell within a radius of 5 m around the parent palm. Two species of beetles were identified as the main seed predators (Table 2). The first was an adult Col.: Curculionidae, Scolytinae (Coccotrypes sp.), which drills through the endocarp. Its entrance hole is characterized by white dust around it, a small perforation size and absence of exudates. The second infesting agent was a beetle larva of Curculionidae. Seeds infested by these larvae were characterized by a larger hole of entrance (ca. 4 - 5 mm
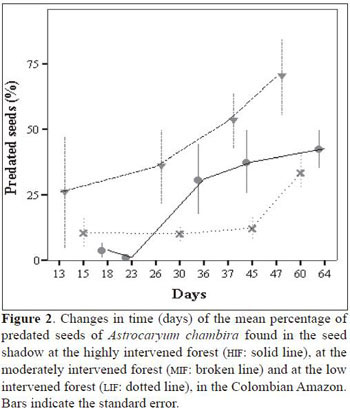
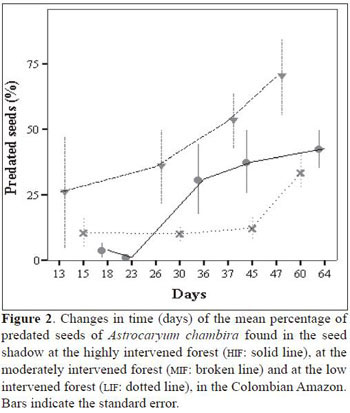
diameter), a foul-smelling brown fluid and yellow or orange exudate outside the fruit or seed, either on the endocarp or the exocarp. The percentage of seeds predated by rodents was very low (Table 2). The percentage of predated seeds over time per forest showed a tendency to increase (Figure 2). Seeds at the HIF suffered a high predation rate within the first 20 days after they fell to the ground, but afterwards, the predation rate decreased. At the MIF, most of the seeds fell to the ground already infested and the rate of predation, once on the ground, was also high. Predation of seeds at the LIF was low and constant in comparison to the other two forests until the 45 th day when the predation rate increased (Figure 2). On the 45th day, there were significant differences in the percentage of predated seeds between forest types (F2,9 = 6.4, P = 0.019). The Tukey HSD test showed that LIF had a significant lower per centage of predated seeds than the MIF (mean difference = -55.4, P = 0.015), but between the MIF and the HIF no significant differences were found (mean difference = 30.1, P = 0.19), nor between the LIF and the HIF (mean difference = 25.4, P = 0.28; Figure 2).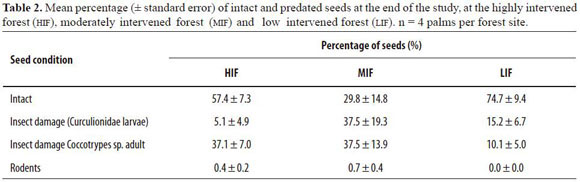
SEED SURVIVAL ANALYSIS
The Cox proportional hazard analysis of the seeds placed at different densities and distances from the parent palm indicated that density does not affect potential survival (five seed-density: Wald 2= 3.3, P = 0.20, two seed-density: Wald1= 0.2, P = 0.65, one seed-density: Wald1 = 3.3, P = 0.71). Therefore, seed density was excluded from the model. The omnibus test of model coefficients indicated that the model including forest type and distance as categorical values was highly significant χ24= 30.5, P < 0.001). Hence, distance affected seed survival. Seeds at 10 m (Wald2= 7.8, P = 0.02) showed a
significantly higher chance of survival than seeds at 2 m (Wald1= 7.7, P = 0.006, HR = 1.6) and than seeds at 50 m (Wald1 = 4.1, P = 0.044, HR = 1.5). Seeds at 2 m and at 50 m had no difference in survival chance (Wald1 = 0.3, P = 0.59). The forest type was significantly associated with seed survival (Figure 3). Seeds at the LIF (Wald2= 21.9, P <0.001) had a significant higher probability of survival than seeds at the MIF (Wald1 = 19.8, P < 0.001, HR= 2.4) and at the HIF (Wald1= 18.6, P <0.001, HR = 2.3). Seeds at the MIF and at the HIF had no significant difference in survival probability (Wald1 = 0.02, P = 0.89). Mean seed survival time at the three forests, obtained through the Kaplan- Meier removal analysis, was the shortest at the HIF(33.1 days ± 0.99), followed by the MIF (32.5 days ± 1.39), while seeds at the LIF exhibited the longest survival time (43.9 days ± 1.24).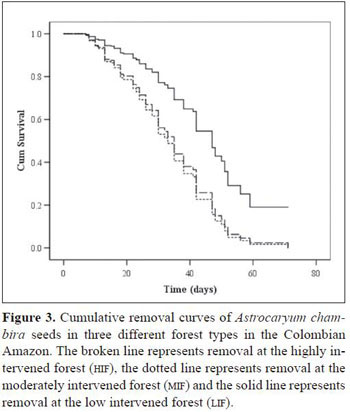
FATE OF MARKED SEEDS
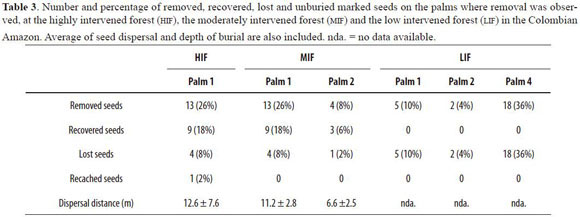
From the four studied palms per forest, seed removal was detected only in one palm of the HIF , two palms of the MIF and three of the LIF. Percentages of seed removal per palm at the HIF was of 26%, at the MIF ranged from 8% to 26%, while at the LIF ranged from 4% to 36%. Dispersal distances were similar between the HIF and the MIF (Table 3). We could not collect data of dispersal distance at the LIF because none of the removed seeds could be recovered as the nylon line was always found cut on the spot.
All seeds at the HIF and MIF started to be removed after the second month of being placed, and all of them exhibited Coccotrypes holes before being removed. This was not the case at the LIF, where all seeds were removed within the first two months after being placed at the stations, and only two of them had holes of Coccotrypes before being removed. In this forest type, we found no evidence of seed predation by rodents such as pieces of seeds that indicated handling. Thus, we assumed all removed seeds were not eaten in situ. There was only one case of recaching of seeds by dispersers at the HIF forest (Table 3).
DISCUSSION
This study provides some evidence on the effects of human intervention on the seed dispersal processes of plants, as we found a strong correspondence between seed removal of Astrocaryum chambira, seed disperser densities and human intervention. First, despite the very small sample size, density of A. chambira seed dispersers such as Myoprocta sp. and D. fuliginosa corresponded to the level of disturbance. Thus, the lowest density was found at the HIF site, followed by the MIF and LIF. Although these differences can be also a consequence of habitat quality, forest structure and floristic composi tion, results coincide with other studies that have found significant differences in vertebrate popula tion densities between heavy, light and non hunted (undisturbed) forest sites (Peres & Palacios 2007). Additionally, even if the number of individuals observed were small, these are likely to be higher than several studies focused on densities and movement patterns for any members of genus Dasyprocta Haugaasen & Peres 2005, Silvius & Fragoso 2003). Since most of the A. chambira seeds remain beneath the parent palm, seed dispersal is rather limited and will exclusively depend on secondary seed dispersers to transport the seeds away, our reported differences in disperser densities may have consequences for seed dispersal and seed predation of the palm, as found in other studies (e.g. Beckman & Muller-Landau 2007, Peres & Palacios 2007). Additionally, even if seed predation at the three sites was mainly caused by insects, seed predation increases with intervention, where no burial occurs, and hence, it is not just that few seeds are dispersed, but also that few will survive.
We found that despite the hard woody endocarp of A. chambira seeds, main predators are two insect species. One is a species of the Curculionidae beetle family which seems to infest the seeds even before they fall to the ground, indicating pre-dispersal predation. This represents a loss to the palm, independently of the effectiveness or abundance of secondary dispersal agents, unless they predate infested seeds (Sallabanks et al. 1992). Unfortunately, as seed predation by rodents was very low in all forest types, we did not obtain evidence of predation of infested seeds by these animals. The second insect predator of the A. chambira seeds was another beetle species of the genus Coccotrypes (Curculionidae, Scolytinae). It predated seeds found under the parent palm, as well as the seeds placed in the experimental stations at 2, 10 and 50 m from the palm. Although we recorded rodents burring seeds already infested by this beetle, we could nottest whether seed burial reduced the insect fitness. Janzen (1972) also reported a species of this genus attacking seeds of Euterpe globosa. He stressed there might be an exponential burst of this predator since generation times are too short and only 5% of the progeny was male. Besides these invertebrate predators, monkeys and squirrels have also been seen predating unripe fruits directly removed from the palm (Stevenson et al. 2000b, Parrado-Rosselli unpubl. data). Although they can reduce the fitness of the Curculionidae predator, these animals also affect the palm regeneration.
In all forest types, caviomorph rodents predated very few seeds in situ (< 1%) while they actively removed and buried A. chambira seeds. The preference of rodents to bury seeds instead of eating them under the parent palm and/or in the stations can be due to the morphological and physiological characteristics of the seeds. Mainly, their long dormancy, high fat content and resistance to rotting make them suitable as reserves for lean periods (Xiao et al. 2006b). As a contrasting example, Forget (1996) reported a higher percentage of predation (10%-20%) in situ by rodents on Carapa procera, which is more susceptible to rotting and early germination (Jansen & Forget 2001) than A. chambira.
The total percentage of predated seeds in all forests showed that during the first two months among 30 70% of the seeds were predated. Also, seed predation percentages increased throughout time. As seeds of A. chambira can stand for almost a year before germinating, they are highly susceptible to reach a 100% of predation if left unburied. Therefore, dispersal and burial by mammals will be very important for the successful regeneration of the palm. Additionally, as predation increases with time, the time frame in which seeds can be removed and buried by rodents before being already infested by insects will vary with human intervention.
Almost every seed of the density and distance experiment was either predated by Coccotrypes or removed by rodents. Regarding density we did not find any effect potential seed survival (see Cox proportional hazard analysis). However, we are aware that our densities were low, and perhaps a higher concentration of seeds (> 5) might have a positive effect on attracting rodents. On the other hand, we did find an effect of distance potential seed survival, as seeds placed at 10 m from the parent palm exhibited a higher removal probability than seeds at 2 m and at 50 m. Probably, seeds at shorter distances will be more exposed to predation because of a higher concentration of seed predators beneath the parent palm (Janzen 1971). In contrast, considering that predation by insects increase with time, and that rodents seem to take more time to find seeds placed at the 50 m stations, the low survival probability of seeds placed at long distances can be due to a broader time window for seeds to be attacked by predators before they are removed or buried. The higher probability of survival at intermediate distances from the parent palm (10 m) coincides with the average dispersal distances obtained in our marked seeds experiment (6 m and 12 m). Thus, it is likely that rodents have intense activity at these distances from the parent palm where they can easily find and bury seeds. These patterns of over dispersion have been reported, for other rodent species, to be effective in reducing cache loss (Male & Smulders 2007). Also, this short distance dispersal and its importance in forest structure has been reported for Dasyprocta leporina in Brazil (Silvius & Fragoso 2003).
Considering the patterns of seed predation and removal described above we can conclude that caviomorph rodents are playing an important role on seed removal. They are high quality dispersers ( sensu Jordano & Schupp 2000) of A. chambira seeds as they do not predate a significant amount of seeds, and because without their removal and burial, seeds will most likely be predated by in sects either beneath the parent palm (Forget & Jansen 2007) or anywhere on the surface of the forest floor. The LIF showed higher seed removal and higher disperser densities than the other two forests (MIF and HIF). Also, there were not significant differences between them both in seed removal and disperser densities. Besides, all marked seeds removed at the HIF and MIF were already infested by Coccotrypes, while at the LIF most seeds were taken intact. The faster removal at the LIF than at the other two forests gives insects a narrower time window to attack. Although, it has been suggested that seed marking delay seed removal (Xiao et al. 2006a); we consider that results are comparable between forests sites.
From a conservation point of view, we conclude that seed removal and consequent burial is crucial for A. chambira seed survival, thus the reduction of seed disperser populations might have a negative impact on the fitness of this species. Considering the importance of A. chambira for local communities, particularly since exploitation depends exclusively on the natural population of the palm, it would be important to maintain adequate densities of its seeds dispersers in order to have favorable regeneration rates and to avoid significant seed loss in natural stands. Fauna management strategies that do not prohibit hunting but that include sustainable harvest levels will be important for enhancing natural regeneration of the species and to avoid depletion in the proximity of local communities. Additionally, palm cultivation can be a strategy, if predation by Coccotrypes is controlled. However, in order to design effective management plans for palm, more information is needed on dispersal distances and its relationship with seedling establishment and recruitment rates. Also, it will be necessary to test whether changes in herbivore populations as a consequence of human disturbance might also affect seedling survival (Terborgh et al. 2008), as well as thresholds caused by human extraction of the subsequent life stages.
CONCLUSIONS
There was a strong correspondence between seed removal of Astrocaryum chambira, seed disperser densities and human intervention.
Most of the A. chambira seeds remained beneath the parent palm; thus seed dispersal exclusively depends on secondary dispersers to transport the seeds away. In consequence, changes in disperser densities might affect the regeneration patterns of the species.
Seed predation increased with intervention, particularly seed predation by Coccotrypes sp. In contrast, seed predation by vertebrates was minimum in all forest sites with no particular pattern.
The importance of caviomorph rodents as seeds dispersers consists on their seed burial. As A. chambira seeds can stand for almost a year before germinating, they are highly susceptible to reach a 100% of predation if left unburied. Additionally, as predation increases with time, the time frame in which seeds can be removed and buried by rodents before being already infested by insects will vary with human intervention.
Fauna management strategies should consider sustainable harvest levels of scatter hoarding rodents, while palm management should consider intermediate establishment distances, as potential seed survival was the highest at 10 m distance from the parent palm. Also Coccotrypes seed predation should be controlled.
ACKNOWLEDGEMENTS
We thank the inhabitants of the community of Macedonia, Centro Ambiental La Pedrera and Fundación Proaves. Willem Hogenes at the Zoölogish Museum, Amsterdam, for the taxonomic identification of the insect larvae. We are also grateful to Patrick Jansen, Andres Link and Jaime Navarro for revising earlier versions of this manuscript. This study was funded by Tropenbos-International Colombia and Fundación Proavesb.
BIBLIOGRAPHIC REFERENCES
Beckman, N. G. & H. C. Muller-Landau. 2007. Differential effects of hunting on pre-dispersal seed predation and primary and secondary seed removal of two neotropical tree species. Biotropica 39: 328-339.
Bodmer, R. E., R. Aquino, P. E. Puertas, C. J. Reyes, T. G. Fang & N. L. Gottdenker. 1997. Manejo y uso sustentable de pecaríes en la Amazonía peruana. Comisión de Supervivencia de Especies. Lima, Perú. Pg. 102.
Borgoft, P. 1994. Mocora palm-fibers: Use and line-management of Astrocaryum standleyanum (Arecaceae) in Ecuador. Economic Botany 48: 310-325.
Burret, M. 1934. Die palmengattung Astrocaryum GFW Myer Repertorium Specierum. Novarum Regni Vegetabilis 35: 114-158.
Bustamante, R. O. & J. A. Simonetti. 2000. Seed predation and seedling recruitment in plants: the effect of the distance between parents. Plant Ecology 147: 173-183.
Calderón, E., G. Galeano & N. García. 2005. Libro rojo de plantas de Colombia. Volumen 2: Palmas, frailejones y zamias. Serie Libros Rojos de Especies Amenazadas de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Instituto de Ciencias Naturales Universidad Nacional de Colombia, Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Bogotá.
Carrillo, E., G. Wong & A. D. Cuarón. 2000. Monitoring mammal populations in Costa Rican Protected Areas under different hunting restrictions. Conservation Biology 14: 1580-1591.
Coomes, O.T. 2004. Rain forest 'conservation through-use'? Chambira palm fibre extraction and handicraft production in a land-constrained community, Peruvian Amazon. Biodiversity and Conservation 13: 351-360.
Cordeiro, N. J. & H. Howe. 2001. Low recruitment of trees dispersed by animals in African forest fragments. Conservation Biology 15 (6): 1733-1741.
Cox, D. R. 1972. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 34:187-220.
De La Hoz, N. 2007. Uso de fauna silvestre por las comunidades indígenas. En: Ruiz S. L., E. Sánchez, E. Tabares, A. Prieto, J. C. Arias, R. Gómez, D. Castellanos, P. García, L. Rodríguez (eds). Diversidad biológica y cultural del sur de la Amazonia colombiana - Diagnóstico. Bogotá. Pp. 356-358.
Defler, T. R. 1996. Aspects of the ranging patterns in a group of wild woolly monkeys (Lagothrix lagotrhicha). American Journal of Primatology 38: 289-302.
Forget, P. M. 1990. Seed-dispersal of Vouacapoua americana (Caesalpiniaceae) by Caviomorph Rodents in French Guiana. Journal of Tropical Ecology 6: 459-468.
Forget, P. M. 1996. Removal of seeds of Carapa procera (Meliaceae) by rodents and their fate in rainforest in French Guiana. Journal of Tropical Ecology 12: 751-761.
Forget, P. M. & P. A. Jansen. 2007. Hunting increases dispersal limitation in the tree Carapa procera, a non-timber forest product. Conservation Biology 21: 106-113.
Forget, P. M. & T. Milleron. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87: 596-599.
Giles, R. 1974. Wildlife management techniques. The Wildlife Society. Washington D.C. Pg. 633.
Haugaasen, T. & C. A. Peres. 2005. Mammal assemblage structure in Amazonian flooded and unflooded forests. Journal of Tropical Ecology 21: 133-145.
Henderson, A. G.R. Galeano, & R. Bernal. 1995. Field guide to the palms of the Americas. Princeton University Press. Princeton, New Jersey.
Henderson, A. 1997. The palms of the Amazons. Oxford University Press. Oxford, UK.
Jansen, P. A. 2003. Scatterhoarding and tree regeneration. Ecology of nut dispersal in a neotropical rainforest. Ph. D. thesis. Wageningen University. Wageningen.
Jansen, P. A. & P. M. Forget. 2001. Scatterhoarding and tree regeneration. En: Bongers, F., P. Charles-Dominique & P. M. Forget (eds.). Nouragues: dynamics and plant-animal interactions in a neotropical rainforest. Kluwer Academic Publishers. Ámsterdam. Pp. 275-288.
Janzen, D. H. 1971. Seed predation by animals. Annual Review of Ecology and Systematics 2: 465-492.
Janzen, D. H. 1972. Association of a rainforest palm and seed-eating beetles in Puerto Rico. Ecology 53: 258-261.
Jensen, O. H. & H. Balslev. 1995. Ethnobotany of the fiber palm Astrocaryum chambira (Arecaceae) in Amazonian Ecuador. Economic Botany: 309-319.
Jordano, P. & E. W. Schupp. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecological Monographs 70: 591-615.
Kahn, F. & F. Moussa. 1994. Diversity and conservation status of Peruvian palms. Biodiversity and Conservation 3: 227-241.
Kleinbaum, D. G. 1996. Removal analysis: a self-learning text. Springer. Nueva York.
Male, L. H. & T. V. Smulders. 2007. Hyperdispersed cache distributions reduce pilferage: a field study. Animal Behaviour 73: 717-726.
Nathan, R. & H. C. Muller-Landau. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology & Evolution 15: 278-285.
Palacios, E. A. & A. Rodríguez. 1995. Caracterización de la dieta y comportamiento alimentario de Callicebus torquatus lugens. Facultad de Ciencias, Departamento de Biología, Universidad Nacional de Colombia. Bogotá.
Peres, C. A. & E. Palacios. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal mediated seed dispersal. Biotropica 39: 305-315.
Pinilla, M. C. 2004. Uso del paisaje en el sector sur del Parque Natural Nacional Amacayacu (Amazonas, Colombia). Cuadernos de Desarrollo Rural 53: 133-156.
Pollock, K.H., S.R. Winterstein, C.M. Bunck & P.D. Curtis. 1989. Removal analysis in telemetry studies: The staggered entry design. The Journal of Wildlife Management 53: 7-15.
Rudas, A. & A. Prieto. 2004. Flórula del Parque Nacional Natural Amacayacu. Missouri Botanical Garden Press. San Luis, Missouri.
Sallabanks, R. & S. P. Courtney. 1992. Frugivory, seed predation, and insect-vertebrate interactions. Annual Review of Entomology 37: 377-400.
Schultes, R. E. 1977. Promising structural fiber palms of the Colombian Amazon. Principes 21: 72-82.
Smythe, N. 1989. Seed removal in the palm Astrocaryum standleyanum: Evidence for dependence upon its seed dispersers. Biotropica 21: 50-56.
SPSS v.14. 2005. SPSS. Chicago.
Silvius, K. M. & J. M. Fragoso. 2003. Redrumped agouti (Dasyprocta leporina) home range use in an Amazonian forest: implications for the aggregated distribution of forest trees. Biotropica 35: 74-83.
Stevenson, P.R., M.J. Quiñones & M.C. Castellanos. 2000a. Guía de frutos de los bosques del río Duda, Macarena, Colombia. Asociación para la Defensa de La Macarena - IUCN. Bogotá. 650 p.
Stevenson, P. R., M. J. Quiñones & J. A. Ahumada. 2000b. Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica 32: 533-544.
Terborgh, J. G. Núñez-Iturri, N.C.A. Pitman, F.H. Cornejo-Valverde, P. Álvarez, V. Swamy, E. G. Pringle & C. E. T. Paine. 2008. Tree recruitment in an empty forest. Ecology 89: 1757-1768
UAESPNN. 1999. Diagnóstico y estrategias de conservación de las poblaciones de fauna silvestre con mayor presión de caza en el sector sur del Parque Nacional Natural Amacayacu. Informe final. Parque Nacional Natural Amacayacu, U.A.O.y.P. (ed.). Ministerio de Ambiente, Vivienda y Desarrollo Territorial.
Vormisto, J. 2002. Making and marketing chambira hammocks and bags in the village of Brillo Nuevo, Northeastern Peru. Economic Botany 56: 27-40.
Xiao, Z., P. A. Jansen & Z. Zhang. 2006a. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. Forest Ecology and Management 223: 18-23.
Xiao, Z., Y. Wang, M. Harris & Z. Zhang. 2006b. Spatial and temporal variation of seed predation and removal of sympatric large-seeded species in relation to innate seed traits in a subtropical forest, Southwest China. Forest Ecology and Management 222: 46-54.
SEED DISPERSAL OF A USEFUL PALM (Astrocaryum chambira Burret) IN THREE AMAZONIAN FORESTS WITH DIFFERENT HUMAN INTERVENTION
Dispersión de semillas de la palma útil (Astrocaryum chambira Burret) en tres bosques amazónicos con diferente grado de intervención humana
Dispersão de sementes da palma útil (Astrocaryum chambira Burret) nos três bosques amazônicos com diferentes graus da intervenção humana
Beatriz H. Ramírez1,2, Ángela Parrado-Rosselli3 & Pablo Stevenson1
1Laboratorio de Ecología de Bosques Tropicales y Primatología,
Centro de Investigaciones Ecológicas La Macarena, Departamento de Ciencias Biológicas
, Universidad de los Andes, AA. 4976, Bogotá,
pstevens@uniandes.edu.co
2Institute of Biodiversity and Ecosystem Dynamics (IBED), Universiteit van Amsterdam.
3Grupo Uso y Conservación de la Diversidad Forestal, Proyecto Curricular de
Ingeniería Forestal, Facultad del Medio Ambiente y Recursos Naturales, Universidad
Distrital Francisco José de Caldas. Correspondencia:
aparrador@udistrital.edu.co
Recepción: Mayo 27 de 2009/Aprobación: Septiembre 5 de 2009
ABSTRACT
The young leaves of Astrocaryum chambira are used by the indigenous people in the Amazon as raw material for handicrafts. However, few studies have been made on the natural history of this palm and on the indirect impact caused by the decrease of its dispersal agents. Considering that the loss of animal dispersal vectors due to hunting and landscape modification can affect seed dispersal proces ses of tropical forest plants, the goal of this study was to compare seed dispersal of A. chambira in three terra firme forests of the Colombian Amazon, with different degrees of human intervention. We censused densities of dispersal agents of A. chambira, and characterized the seed shadow. We also marked seeds to estimate dispersal distances, and established density and distance-dependent experimental stations to assess their relevance on seed dispersal. The results showed that seed removal was proportional to dispersal agent densities and forest intervention levels. Insects were the main seed predators in all sites but their effect was less pronounced in the low intervened forest site. Seed density did not show any effect on removal, while a higher probability of survival at intermediate distances from the parent palm (10 m) was found. Future studies should focus on seedling establishment, recruitment rates and the effects of human intervention on subsequent life stages of the palm.
Key words: Amazon forest, chambira palm, seed predation, insect seed predation, rodents.
RESUMEN
Las hojas jóvenes de Astrocaryum chambira son utilizadas por las comunidades indígenas amazónicas como materia prima para la fabricación de artesanías. Sin embargo, son muy pocos los estudios acerca de su historia de vida y de los impactos indirectos causados por la disminución de sus agentes dispersores. Teniendo en cuenta que la pérdida de animales dispersores de semillas por factores como cacería y modificación de hábitat afecta la dispersión de semillas de las especies de plantas tropicales, el objetivo de este estudio fue comparar la dispersión de semillas de A. chambira en tres bosques de tierra firme del Amazonas colombiano sujetos a diferentes niveles de intervención antrópica. Censamos las densidades de los agentes dispersores de A. chambira y caracterizamos la sombra de semillas. También marcamos semillas con el fin de estimar las distancias de dispersión y establecimos estaciones experimentales de densodistancio-dependencia para evaluar su relevancia en la dispersión de semillas de esta especie. Los resultados muestran que la remoción de semillas fue proporcional a la densidad de animales y al nivel de intervención del bosque. Los insectos fueron los principales depredadores en todos los sitios pero su efecto fue menos pronunciado en el bosque me-nos intervenido. La densidad de semillas no generó ningún efecto en la remoción, mientras que encontramos una mayor probabilidad de supervivencia a distancias intermedias de la palma (10 m). Estudios futuros se deberían enfocar en el establecimiento de las plántulas, las tasas de reclutamiento y el efecto de la intervención antrópica en los posteriores estadios de vida de esta palma tropical.
Palabras clave: bosque amazónico, depredación de semillas, depredación por insectos, palma de chambira, roedores.
RESUMO
As folhas jóvens de Astrocaryum chambira são utilizadas pelas comunidades indígenas amazônicas como matéria prima para a fabricação de artesanato. Sem embargo, são muito poucos os estudos acerca da sua historia de vida e dos impactos indiretos causados pela diminuição dos seus agentes dispersores. Tendo em conta que a perda de animais dispersores de sementes por fatores como a caça e a modificação de hábitat afeta a dispersão de sementes das espécies de plantas tropicais, o objetivo deste estudo foi comparar a dispersão de sementes de A. chambira em três bosques de terra firme do Amazonas colombiano sujeitos a diferentes níveis de intervenção antrópica. Censamos as densidades dos agentes dispersores de A. chambira e caracterizamos a sombra de sementes. Também marcamos sementes com a finalidade de estimar as distâncias de dispersão e estabelecer estações experimentais de denso distância-dependência para avaliar sua relevância na dispersão de sementes desta espécie. Os resultados mostram que a remoção de sementes foi proporcional a densidade dos animais e ao nível de intervenção do bosque. Os insetos foram os principais depredadores em todos os lugares, mas seu efeito foi menos pronunciado nos bosque não intervenidos. A densidade de sementes não gerou nenhum efeito na remoção, enquanto que encontramos una maior probabilidade de sobrevivência às distâncias intermediárias da palma (10 m). Estudos futuros deveríam se enfocar no estabelecimiento das mudas, as taxas de recrutamento e o efeito da intervenção antrópica nos posteriores estágios de vida desta palma tropical.
Palavras chave: Floresta Amazônica, depredação das sementes, depredação pelos insetos, palma de chambira, roedores.
INTRODUCTION
The chambira palm, Astrocaryum chambira Burret (1934) (Arecaceae), has been traditionally used by different indigenous communities of Amazonia (Schultes 1977, Jensen & Balslev 1995, Vormisto 2002). They obtain fibers from new leaves to produce nets, hammocks, household and ritual artifacts (Borgoft 1994, Jensen & Balslev 1995, Vormisto 2002). These products also generate economical income due to the interest of tourists in local handi-crafts (Jensen & Balslev 1995). The traditional harvest system consists on the removal of the youngest unfolded leaf, but leaving the next one intact in order to guarantee palm growth (Borgoft 1994, Jensen & Balslev 1995). However, it has been reported that several communities are not using this system anymore, harvesting the whole plant, and therefore affecting the development and growth regeneration dynamics of the species (Coomes 2004). Additionally to these direct stresses, other anthropogenic disturbances can alter the demography of the species. For example, the loss of animal dispersal vectors due to hunting and landscape modification can affect seed dispersal processes of tropical forest plants (Beckman & Muller-Landau 2007, Peres & Palacios 2007), which in turn have an effect on recruitment rates, genetic flux, coloni zation abilities and patterns of spatial distribution (Nathan & Muller-Landau 2000). Since trade of handicrafts has been increasing, but the exploitation depends exclusively on the natural population of the palm (Coomes 2004), it is unknown whether the natural populations of A. chambira will be able to tolerate such demand.
A. chambira is a palm species dispersed mainly by caviomorph rodents such as species of the genera Agouti, Dasyprocta and Myoprocta. These animals generally act as seed predators as they bury seeds to retrieve and eat them in periods of food scarcity. The successful seed dispersal occurs when the rodents fail to recover the buried seeds (Smythe 1989, Jansen & Forget 2001). The advantage of seed burial is that it reduces seed predation, as insects predate upon unburied seeds, particularly those that are not taken away from the parent palm (Janzen 1971, Forget 1990, Forget & Milleron 1991). The genera Agouti and Dasyprocta, commonly known as “borugos” and “guaras”, respectively, have been highly targeted by hunters. Additionally, although in several places of the Amazon Myoprocta is usually ignored (Carrillo et al. 2000, Peres & Palacios 2007), in the Colombian Amazonia they are highly hunted, particularly in intervened forests where they are found by dogs or caught with small traps by any member of an indigenous community working in the community garden (De la Hoz 2007). Thus, human intervention in the forest, generated by both planned and/or sporadic hunting, and proximity to settlements might be related with low animal population densities and affect regeneration dynamics of the species. For instance, Peres & Palacios (2007) have found lower population densities of caviomorph rodents in heavily hunted forests than in lightly hunted forests or non-hunted forests. Also, changes in mammal densities have shown differential effects on seed dispersal and seed predation depending on the traits and dispersal modes of the plant species (Bustamante & Simonetti 2000, Cordeiro & Howe 2001, Beckman & Muller-Landau 2007). In that way, knowledge of the seed dispersal processes of A. chambira under different intervention conditions might be very important for management purposes of its natural populations. Since A. chambira is one-large-seeded species that depends on secondary seed dispersal, a reduction in seed-disperser populations by hunting or any other form of human disturbance might re duce the proportion of removed seeds, and many of them will remain under the parent plant where the risk of mortality by seed predation is the highest (Forget & Jansen 2007).
In order to contribute to a better understanding of the consequences of changes in disperser populations for regeneration of tropical forest plants, we studied the seed fate of A. chambira in three forests subjected to different degree of human intervention, where densities of seed dispersers were supposed to be different. For this purpose, in each forest site we censused the dispersal agents, characterized the seed shadow and evaluated seed predation, seed removal by animals and seed dispersal distances. With lower disperser densities as a consequence of human intervention, we expected less seeds removed and shorter dispersal distances from the parent palm.
METHODS
STUDY AREA
Three Amazonian Tropical Humid Forest sites were selected for this study. The first two sites were located in Macedonia, an indigenous community on the northern margin of the Amazon River, state of Amazonas, Colombia (70° 13’ 22.4” W, 3° 49’ 01.0” S). Mean annual temperature is 26ºC and mean annual precipitation reaches values of 3200 mm, averaging 270 mm per month (Rudas & Prieto 2004). July and October are the driest months while January and April are the wettest months (UAESPNN 1999). The two sites were selected on the basis of the degree of human intervention according to Pinilla (2004) from high to moderate. The highly intervened forest (HIF) was considered as the forest continuously used by indigenous people for the extraction of both timber and non timber products (Pinilla 2004), including A. chambira leaves for craft-making purposes. This forest was located less than 5 km away from the community (modi fied from Pinilla 2004), where local people have used slash-and-burn cultivation systems, which have turned the forest into a mosaic of different regeneration stages. The moderately intervened forest (MIF ) consisted on forests regularly used for hunting and natural resources harvesting (planned journeys), including the extraction of A. chambira leaves, neither historic nor current cultivation. For the purposes of this study, this site was located more than 5 km away from the Macedonia settle ment (modified from Pinilla 2004).
The third forest was located at the biological station Mosiro Itajura located in the state of Vaupés, Colombia (69°31’2.9’’W, 1°04’21.8’’S). Mean annual temperature is 25.1° C and mean annual precipitation is between 3000 and 4000 mm (De fler 1996). May is the month with the highest precipitation (384 mm) and September is the driest month (258 mm: Palacios & Rodríguez 1995). The nearest indigenous community is located more than 8 km away in a straight line or more than 5 hours by foot. According to the local indigenous communities this is a sacred site, therefore hunting has always been restricted. Also, the existence since the 1980s of the biological station has entailed additional protection. There is no exploitation of young leaves of A. chambira or harvest of any other forest products. This forest was defined as the low intervened forest (LIF).
STUDY SPECIES
Astrocaryum chambira is a palm with a solitary erect trunk up to 22 m tall and 19-35 cm in dia meter (Henderson et al. 1995). The internodes are covered with black or grey spines of up to 20 cm in length. The leaf rachis is covered densely by yellow or brown flat spines of 3 up to 15 cm in length. Four to six leaves can be produced per year (Coomes 2004). Inflorescences are interfoliary and erect at anthesis, and in fruit. Fruits are obovoid of 5-6 cm in length by 4-4.5 cm in diameter, with yellow-green epicarp and with tiny spinules of white-brown color. The mesocarp is fibrous and yellow when ripe (Stevenson et al. 2000a). The endocarp is black, thick and bony with three lateral pores (Henderson et al. 1995). Each fruit is single-seeded and according to Bodmer et al. (1997) both fruits and seeds of the genus Astrocaryum are lipid-rich. Germination of the seeds occurs 8-10 months after they fall to the ground (Coomes 2004).
A. chambira is restricted to the Amazon basin and it can be found in terra firme , open vegetation and temporarily flooded forests (Várzea). It can be highly frequent at elevations below 350 m, and it is not categorized as an endangered species (Kahn & Moussa 1994, Henderson 1997, Calderón et al. 2005). A. chambira is distributed along the Amazon region of Colombia (Amazonas, Caque tá, Guaviare, Meta, Putumayo and Vaupés), Venezuela (Amazonas), Ecuador (Morona-Santiago, Napo), Perú (Amazonas, Loreto) and Brazil (Acre, Amazonas) (Henderson et al. 1995).
DATA COLLECTION
Data were collected from January to May 2004 at the HIF and the MIF, and from February to June 2005 at the LIF. In each forest we estimated density of seed dispersal agents, and chose eight fruiting individuals of A. chambira to characterize the seed shadow, seed removal and dispersal distances. The minimum distance between individuals was 100 m.
Census of seed dispersal agents
In each forest type, we estimated the density of dispersal agents of A. chambira, Dasyprocta fuliginosa (common name guara) and Myoprocta sp. (Myoprocta acouchy - Myoprocta exilis; common name tintín) by visual censuses on linear transects. The total length covered by the census was 60.6 km at the HIF, 56.7 km at the MIF and 129.4 km at the LIF. The effective transect width was determined by King’s method (Giles 1974). Although forests have been subjected to a different disturbance regime, altering both plant and animal species composition, there were not significant more open conditions at the HIF, as well as we did not sample in gardens or barbechs. Additionally, effective transect width was estimated based on the perpendicular distance from the centre of the survey path separately in each forest site, where the largest width was obtained at the LIFsite (mean 12.04 m). Thus, animal censuses were not biased in favor of a particular site. The average speed of the census was 2 km/h (SD = 0.3 km/h). Censuses were carried out over 3 months at least once per week, between 6:00 h and 10:00 h. Each time a D. fuliginosa or Myoprocta sp. was observed, the perpendicular distance to the transect and the number of individuals were recorded. The Kruskall-Wallis analysis was used in order to compare densities of each rodent species between forest types. Also, in order to define the differences between pair of forests Mann-Whitney U-tests were performed.
Seed shadow
Four of the eight adult individuals of A. chambira selected per forest were used to characterize the seed shadow created after the fruits fell from the palm. Around the trunk of each individual we established a 78.5 m2 area (5 m radius). From this 5 m radius we set six transects of 1 m × 50 m every 60°. Every fifteen days, we counted and classified all A. chambira seeds found within the 78.5 m 2 area and in each transect. Seeds were classified according to the seed’s condition: intact, predated by mam mals, or predated by insects. A seed was classified as intact when its endocarp was not perforated or broken. Evidence to classify a seed as predated by mammals consisted of broken seeds or pieces of endocarp found on the ground. When a seed presented holes that passed through the endocarp and reached the endosperm it was classified as predated by insects. In this case, these were classified into subgroups depending on the infesting agent. Samples of the predating insects were identified at the entomological collection of the Amsterdam Zoological Museum.
One-way analysis of variance (ANOVA) was performed to compare the differences between forests in the amount of fruits found in the 5 m radius area around each palm. Change in the percentage of predated seeds along time was obtained for forest site. We compared the proportion of predated seeds per forest at the 45th day after they began to fall to the ground. If there were significant differences we carried out a post hoc Tukey HSD test. All analyses were performed using the software SPSS version 14 (SPSS 2005).
Seed removal and dispersal distance
The other four individuals of A. chambira per forest site were used to experimentally assess seed removal by animals, dispersal distance and densi-transect had a different combination of distance ty and distance-dependent effects. In each palm, and density treatments (Table 1). On a biweekly every 72° we installed five 50 m transects from the basis, we counted the number of seeds per station trunk. In each transect we placed three stations at and determined their condition (intact, predated or 2, 10 and 50 m from the palm. On each station, missing). We never replaced predated or missing densities were of one, two and five seeds. Each seeds.
The Cox proportional hazard analysis (Cox 1972) was used to determine if the forest type, the distance from the parent palm or the seed density significantly affected potential seed survival. It was calculated as those seeds that remained intact in the station and/or those that were missing (assuming that removal enhances seed survival) minus those seeds predated. All variables were treated as cate gorical values. If differences were significant we showed the Hazard Ratio (HR), which indicates the increase in the probability of a seed to potentially survive when subjected to a particular treatment in comparison to the other one. To test for the proportional hazard assumption we visually examined the Log-Log plots for parallel curves (Kleinbaum 1996). We also estimated the mean removal time of the seeds at each forest type with a Kaplan-Meier removal analysis (Pollock et al. 1989). Analyses were made using SPSS version 14 (SPSS 2005).
For determining dispersal distances we marked 50 seeds per palm, all of them with green epicarp and yellow mesocarp. We marked each seed by attaching 50 cm of nylon line with 10 cm of vinyl fluo rescent tape (sensu Jansen 2003). To tie the seed, we drilled a hole through the tip of the seed without entering the endosperm and passed the nylon line through it. Due to the lack of electricity at the LIF forest, we used glue to attach the nylon thread to the seed. Seeds were placed in ten groups of five seeds at 3 m distance from the parent trunk and checked them every 15 days. If a seed was missing we looked for it along ten transects 50 m long that began from the trunk of the palm every 36°. For each seed found we measured the dispersal distance and depth. We also verified whether removed seeds found on previous revisions were still buried, and their condition was recorded only until the end of this study. Non-recovered seeds were classified as lost.
RESULTS
CENSUSES OF DISPERSAL AGENTS
Mean density of D. fuliginosa individuals at the HIFwas 7 ind/km2, and 10 ind/km 2 at the MIF and at the LIF. Mean density of Myoprocta sp. at the HIF was 5 ind/km2, 11 ind/km2at the MIF and 16 ind/km2 at the LIF. Total D. fuliginosa and Myoprocta sp. sample size were 45 and 54 individuals in all forest sites, respectively. Although the number of individuals observed was small, the analysis showed significant differences in densities of Myoprocta sp. and D. fuliginosa among forests (D. fuliginosa: χ−22 = 9.4, P = 0.009, Myoprocta sp.:χ−22= 16.3, P < 0.001; Figure 1). Differences between the HIF and the MIF were not significant for both rodent species ( D. fuliginosa: Z = -0.3, P = 0.762, Myoprocta sp: Z = -0.5, P = 0.650). In contrast, we found significant differences between the LIF and the MIF for both D. fuliginosa and Myoprocta sp. (Z = -2.2, P = 0.029 and Z = -3.4, P = 0.001, respectively), and between the LIFand the HIF as well (D. fuliginosa: Z = -2.2, P = 0.008, Myoprocta sp: Z = -3.0, P = 0.003).
SEED SHADOWS AND SEED PREDATION
Our results showed that all seeds produced by A. chambira palms in the three forest sites fell within a radius of 5 m around the parent palm. Two species of beetles were identified as the main seed predators (Table 2). The first was an adult Col.: Curculionidae, Scolytinae (Coccotrypes sp.), which drills through the endocarp. Its entrance hole is characterized by white dust around it, a small perforation size and absence of exudates. The second infesting agent was a beetle larva of Curculionidae. Seeds infested by these larvae were characterized by a larger hole of entrance (ca. 4 - 5 mm
diameter), a foul-smelling brown fluid and yellow or orange exudate outside the fruit or seed, either on the endocarp or the exocarp. The percentage of seeds predated by rodents was very low (Table 2). The percentage of predated seeds over time per forest showed a tendency to increase (Figure 2). Seeds at the HIF suffered a high predation rate within the first 20 days after they fell to the ground, but afterwards, the predation rate decreased. At the MIF, most of the seeds fell to the ground already infested and the rate of predation, once on the ground, was also high. Predation of seeds at the LIF was low and constant in comparison to the other two forests until the 45 th day when the predation rate increased (Figure 2). On the 45th day, there were significant differences in the percentage of predated seeds between forest types (F2,9 = 6.4, P = 0.019). The Tukey HSD test showed that LIF had a significant lower per centage of predated seeds than the MIF (mean difference = -55.4, P = 0.015), but between the MIF and the HIF no significant differences were found (mean difference = 30.1, P = 0.19), nor between the LIF and the HIF (mean difference = 25.4, P = 0.28; Figure 2).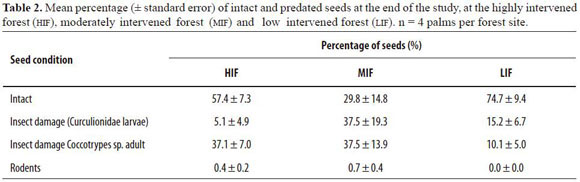
SEED SURVIVAL ANALYSIS
The Cox proportional hazard analysis of the seeds placed at different densities and distances from the parent palm indicated that density does not affect potential survival (five seed-density: Wald 2= 3.3, P = 0.20, two seed-density: Wald1= 0.2, P = 0.65, one seed-density: Wald1 = 3.3, P = 0.71). Therefore, seed density was excluded from the model. The omnibus test of model coefficients indicated that the model including forest type and distance as categorical values was highly significant χ24= 30.5, P < 0.001). Hence, distance affected seed survival. Seeds at 10 m (Wald2= 7.8, P = 0.02) showed a
significantly higher chance of survival than seeds at 2 m (Wald1= 7.7, P = 0.006, HR = 1.6) and than seeds at 50 m (Wald1 = 4.1, P = 0.044, HR = 1.5). Seeds at 2 m and at 50 m had no difference in survival chance (Wald1 = 0.3, P = 0.59). The forest type was significantly associated with seed survival (Figure 3). Seeds at the LIF (Wald2= 21.9, P <0.001) had a significant higher probability of survival than seeds at the MIF (Wald1 = 19.8, P < 0.001, HR= 2.4) and at the HIF (Wald1= 18.6, P <0.001, HR = 2.3). Seeds at the MIF and at the HIF had no significant difference in survival probability (Wald1 = 0.02, P = 0.89). Mean seed survival time at the three forests, obtained through the Kaplan- Meier removal analysis, was the shortest at the HIF(33.1 days ± 0.99), followed by the MIF (32.5 days ± 1.39), while seeds at the LIF exhibited the longest survival time (43.9 days ± 1.24).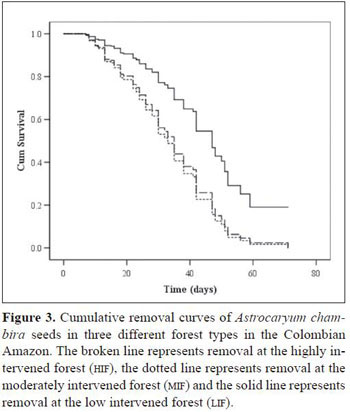
FATE OF MARKED SEEDS
From the four studied palms per forest, seed removal was detected only in one palm of the HIF , two palms of the MIF and three of the LIF. Percentages of seed removal per palm at the HIF was of 26%, at the MIF ranged from 8% to 26%, while at the LIF ranged from 4% to 36%. Dispersal distances were similar between the HIF and the MIF (Table 3). We could not collect data of dispersal distance at the LIF because none of the removed seeds could be recovered as the nylon line was always found cut on the spot.
All seeds at the HIF and MIF started to be removed after the second month of being placed, and all of them exhibited Coccotrypes holes before being removed. This was not the case at the LIF, where all seeds were removed within the first two months after being placed at the stations, and only two of them had holes of Coccotrypes before being removed. In this forest type, we found no evidence of seed predation by rodents such as pieces of seeds that indicated handling. Thus, we assumed all removed seeds were not eaten in situ. There was only one case of recaching of seeds by dispersers at the HIF forest (Table 3).
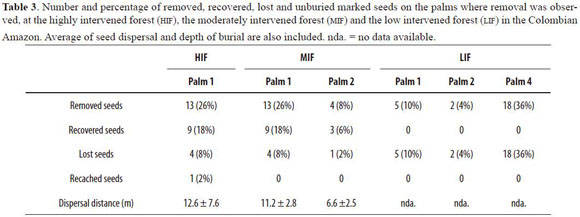
DISCUSSION
This study provides some evidence on the effects of human intervention on the seed dispersal processes of plants, as we found a strong correspondence between seed removal of Astrocaryum chambira, seed disperser densities and human intervention. First, despite the very small sample size, density of A. chambira seed dispersers such as Myoprocta sp. and D. fuliginosa corresponded to the level of disturbance. Thus, the lowest density was found at the HIF site, followed by the MIF and LIF. Although these differences can be also a consequence of habitat quality, forest structure and floristic composi tion, results coincide with other studies that have found significant differences in vertebrate popula tion densities between heavy, light and non hunted (undisturbed) forest sites (Peres & Palacios 2007). Additionally, even if the number of individuals observed were small, these are likely to be higher than several studies focused on densities and movement patterns for any members of genus Dasyprocta Haugaasen & Peres 2005, Silvius & Fragoso 2003). Since most of the A. chambira seeds remain beneath the parent palm, seed dispersal is rather limited and will exclusively depend on secondary seed dispersers to transport the seeds away, our reported differences in disperser densities may have consequences for seed dispersal and seed predation of the palm, as found in other studies (e.g. Beckman & Muller-Landau 2007, Peres & Palacios 2007). Additionally, even if seed predation at the three sites was mainly caused by insects, seed predation increases with intervention, where no burial occurs, and hence, it is not just that few seeds are dispersed, but also that few will survive.
We found that despite the hard woody endocarp of A. chambira seeds, main predators are two insect species. One is a species of the Curculionidae beetle family which seems to infest the seeds even before they fall to the ground, indicating pre-dispersal predation. This represents a loss to the palm, independently of the effectiveness or abundance of secondary dispersal agents, unless they predate infested seeds (Sallabanks et al. 1992). Unfortunately, as seed predation by rodents was very low in all forest types, we did not obtain evidence of predation of infested seeds by these animals. The second insect predator of the A. chambira seeds was another beetle species of the genus Coccotrypes (Curculionidae, Scolytinae). It predated seeds found under the parent palm, as well as the seeds placed in the experimental stations at 2, 10 and 50 m from the palm. Although we recorded rodents burring seeds already infested by this beetle, we could nottest whether seed burial reduced the insect fitness. Janzen (1972) also reported a species of this genus attacking seeds of Euterpe globosa. He stressed there might be an exponential burst of this predator since generation times are too short and only 5% of the progeny was male. Besides these invertebrate predators, monkeys and squirrels have also been seen predating unripe fruits directly removed from the palm (Stevenson et al. 2000b, Parrado-Rosselli unpubl. data). Although they can reduce the fitness of the Curculionidae predator, these animals also affect the palm regeneration.
In all forest types, caviomorph rodents predated very few seeds in situ (< 1%) while they actively removed and buried A. chambira seeds. The preference of rodents to bury seeds instead of eating them under the parent palm and/or in the stations can be due to the morphological and physiological characteristics of the seeds. Mainly, their long dormancy, high fat content and resistance to rotting make them suitable as reserves for lean periods (Xiao et al. 2006b). As a contrasting example, Forget (1996) reported a higher percentage of predation (10%-20%) in situ by rodents on Carapa procera, which is more susceptible to rotting and early germination (Jansen & Forget 2001) than A. chambira.
The total percentage of predated seeds in all forests showed that during the first two months among 30 70% of the seeds were predated. Also, seed predation percentages increased throughout time. As seeds of A. chambira can stand for almost a year before germinating, they are highly susceptible to reach a 100% of predation if left unburied. Therefore, dispersal and burial by mammals will be very important for the successful regeneration of the palm. Additionally, as predation increases with time, the time frame in which seeds can be removed and buried by rodents before being already infested by insects will vary with human intervention.
Almost every seed of the density and distance experiment was either predated by Coccotrypes or removed by rodents. Regarding density we did not find any effect potential seed survival (see Cox proportional hazard analysis). However, we are aware that our densities were low, and perhaps a higher concentration of seeds (> 5) might have a positive effect on attracting rodents. On the other hand, we did find an effect of distance potential seed survival, as seeds placed at 10 m from the parent palm exhibited a higher removal probability than seeds at 2 m and at 50 m. Probably, seeds at shorter distances will be more exposed to predation because of a higher concentration of seed predators beneath the parent palm (Janzen 1971). In contrast, considering that predation by insects increase with time, and that rodents seem to take more time to find seeds placed at the 50 m stations, the low survival probability of seeds placed at long distances can be due to a broader time window for seeds to be attacked by predators before they are removed or buried. The higher probability of survival at intermediate distances from the parent palm (10 m) coincides with the average dispersal distances obtained in our marked seeds experiment (6 m and 12 m). Thus, it is likely that rodents have intense activity at these distances from the parent palm where they can easily find and bury seeds. These patterns of over dispersion have been reported, for other rodent species, to be effective in reducing cache loss (Male & Smulders 2007). Also, this short distance dispersal and its importance in forest structure has been reported for Dasyprocta leporina in Brazil (Silvius & Fragoso 2003).
Considering the patterns of seed predation and removal described above we can conclude that caviomorph rodents are playing an important role on seed removal. They are high quality dispersers ( sensu Jordano & Schupp 2000) of A. chambira seeds as they do not predate a significant amount of seeds, and because without their removal and burial, seeds will most likely be predated by in sects either beneath the parent palm (Forget & Jansen 2007) or anywhere on the surface of the forest floor. The LIF showed higher seed removal and higher disperser densities than the other two forests (MIF and HIF). Also, there were not significant differences between them both in seed removal and disperser densities. Besides, all marked seeds removed at the HIF and MIF were already infested by Coccotrypes, while at the LIF most seeds were taken intact. The faster removal at the LIF than at the other two forests gives insects a narrower time window to attack. Although, it has been suggested that seed marking delay seed removal (Xiao et al. 2006a); we consider that results are comparable between forests sites.
From a conservation point of view, we conclude that seed removal and consequent burial is crucial for A. chambira seed survival, thus the reduction of seed disperser populations might have a negative impact on the fitness of this species. Considering the importance of A. chambira for local communities, particularly since exploitation depends exclusively on the natural population of the palm, it would be important to maintain adequate densities of its seeds dispersers in order to have favorable regeneration rates and to avoid significant seed loss in natural stands. Fauna management strategies that do not prohibit hunting but that include sustainable harvest levels will be important for enhancing natural regeneration of the species and to avoid depletion in the proximity of local communities. Additionally, palm cultivation can be a strategy, if predation by Coccotrypes is controlled. However, in order to design effective management plans for palm, more information is needed on dispersal distances and its relationship with seedling establishment and recruitment rates. Also, it will be necessary to test whether changes in herbivore populations as a consequence of human disturbance might also affect seedling survival (Terborgh et al. 2008), as well as thresholds caused by human extraction of the subsequent life stages.
CONCLUSIONS
There was a strong correspondence between seed removal of Astrocaryum chambira, seed disperser densities and human intervention.
Most of the A. chambira seeds remained beneath the parent palm; thus seed dispersal exclusively depends on secondary dispersers to transport the seeds away. In consequence, changes in disperser densities might affect the regeneration patterns of the species.
Seed predation increased with intervention, particularly seed predation by Coccotrypes sp. In contrast, seed predation by vertebrates was minimum in all forest sites with no particular pattern.
The importance of caviomorph rodents as seeds dispersers consists on their seed burial. As A. chambira seeds can stand for almost a year before germinating, they are highly susceptible to reach a 100% of predation if left unburied. Additionally, as predation increases with time, the time frame in which seeds can be removed and buried by rodents before being already infested by insects will vary with human intervention.
Fauna management strategies should consider sustainable harvest levels of scatter hoarding rodents, while palm management should consider intermediate establishment distances, as potential seed survival was the highest at 10 m distance from the parent palm. Also Coccotrypes seed predation should be controlled.
ACKNOWLEDGEMENTS
We thank the inhabitants of the community of Macedonia, Centro Ambiental La Pedrera and Fundación Proaves. Willem Hogenes at the Zoölogish Museum, Amsterdam, for the taxonomic identification of the insect larvae. We are also grateful to Patrick Jansen, Andres Link and Jaime Navarro for revising earlier versions of this manuscript. This study was funded by Tropenbos-International Colombia and Fundación Proavesb.
BIBLIOGRAPHIC REFERENCES
Beckman, N. G. & H. C. Muller-Landau. 2007. Differential effects of hunting on pre-dispersal seed predation and primary and secondary seed removal of two neotropical tree species. Biotropica 39: 328-339.
Bodmer, R. E., R. Aquino, P. E. Puertas, C. J. Reyes, T. G. Fang & N. L. Gottdenker. 1997. Manejo y uso sustentable de pecaríes en la Amazonía peruana. Comisión de Supervivencia de Especies. Lima, Perú. Pg. 102.
Borgoft, P. 1994. Mocora palm-fibers: Use and line-management of Astrocaryum standleyanum (Arecaceae) in Ecuador. Economic Botany 48: 310-325.
Burret, M. 1934. Die palmengattung Astrocaryum GFW Myer Repertorium Specierum. Novarum Regni Vegetabilis 35: 114-158.
Bustamante, R. O. & J. A. Simonetti. 2000. Seed predation and seedling recruitment in plants: the effect of the distance between parents. Plant Ecology 147: 173-183.
Calderón, E., G. Galeano & N. García. 2005. Libro rojo de plantas de Colombia. Volumen 2: Palmas, frailejones y zamias. Serie Libros Rojos de Especies Amenazadas de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Instituto de Ciencias Naturales Universidad Nacional de Colombia, Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Bogotá.
Carrillo, E., G. Wong & A. D. Cuarón. 2000. Monitoring mammal populations in Costa Rican Protected Areas under different hunting restrictions. Conservation Biology 14: 1580-1591.
Coomes, O.T. 2004. Rain forest 'conservation through-use'? Chambira palm fibre extraction and handicraft production in a land-constrained community, Peruvian Amazon. Biodiversity and Conservation 13: 351-360.
Cordeiro, N. J. & H. Howe. 2001. Low recruitment of trees dispersed by animals in African forest fragments. Conservation Biology 15 (6): 1733-1741.
Cox, D. R. 1972. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 34:187-220.
De La Hoz, N. 2007. Uso de fauna silvestre por las comunidades indígenas. En: Ruiz S. L., E. Sánchez, E. Tabares, A. Prieto, J. C. Arias, R. Gómez, D. Castellanos, P. García, L. Rodríguez (eds). Diversidad biológica y cultural del sur de la Amazonia colombiana - Diagnóstico. Bogotá. Pp. 356-358.
Defler, T. R. 1996. Aspects of the ranging patterns in a group of wild woolly monkeys (Lagothrix lagotrhicha). American Journal of Primatology 38: 289-302.
Forget, P. M. 1990. Seed-dispersal of Vouacapoua americana (Caesalpiniaceae) by Caviomorph Rodents in French Guiana. Journal of Tropical Ecology 6: 459-468.
Forget, P. M. 1996. Removal of seeds of Carapa procera (Meliaceae) by rodents and their fate in rainforest in French Guiana. Journal of Tropical Ecology 12: 751-761.
Forget, P. M. & P. A. Jansen. 2007. Hunting increases dispersal limitation in the tree Carapa procera, a non-timber forest product. Conservation Biology 21: 106-113.
Forget, P. M. & T. Milleron. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87: 596-599.
Giles, R. 1974. Wildlife management techniques. The Wildlife Society. Washington D.C. Pg. 633.
Haugaasen, T. & C. A. Peres. 2005. Mammal assemblage structure in Amazonian flooded and unflooded forests. Journal of Tropical Ecology 21: 133-145.
Henderson, A. G.R. Galeano, & R. Bernal. 1995. Field guide to the palms of the Americas. Princeton University Press. Princeton, New Jersey.
Henderson, A. 1997. The palms of the Amazons. Oxford University Press. Oxford, UK.
Jansen, P. A. 2003. Scatterhoarding and tree regeneration. Ecology of nut dispersal in a neotropical rainforest. Ph. D. thesis. Wageningen University. Wageningen.
Jansen, P. A. & P. M. Forget. 2001. Scatterhoarding and tree regeneration. En: Bongers, F., P. Charles-Dominique & P. M. Forget (eds.). Nouragues: dynamics and plant-animal interactions in a neotropical rainforest. Kluwer Academic Publishers. Ámsterdam. Pp. 275-288.
Janzen, D. H. 1971. Seed predation by animals. Annual Review of Ecology and Systematics 2: 465-492.
Janzen, D. H. 1972. Association of a rainforest palm and seed-eating beetles in Puerto Rico. Ecology 53: 258-261.
Jensen, O. H. & H. Balslev. 1995. Ethnobotany of the fiber palm Astrocaryum chambira (Arecaceae) in Amazonian Ecuador. Economic Botany: 309-319.
Jordano, P. & E. W. Schupp. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecological Monographs 70: 591-615.
Kahn, F. & F. Moussa. 1994. Diversity and conservation status of Peruvian palms. Biodiversity and Conservation 3: 227-241.
Kleinbaum, D. G. 1996. Removal analysis: a self-learning text. Springer. Nueva York.
Male, L. H. & T. V. Smulders. 2007. Hyperdispersed cache distributions reduce pilferage: a field study. Animal Behaviour 73: 717-726.
Nathan, R. & H. C. Muller-Landau. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology & Evolution 15: 278-285.
Palacios, E. A. & A. Rodríguez. 1995. Caracterización de la dieta y comportamiento alimentario de Callicebus torquatus lugens. Facultad de Ciencias, Departamento de Biología, Universidad Nacional de Colombia. Bogotá.
Peres, C. A. & E. Palacios. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal mediated seed dispersal. Biotropica 39: 305-315.
Pinilla, M. C. 2004. Uso del paisaje en el sector sur del Parque Natural Nacional Amacayacu (Amazonas, Colombia). Cuadernos de Desarrollo Rural 53: 133-156.
Pollock, K.H., S.R. Winterstein, C.M. Bunck & P.D. Curtis. 1989. Removal analysis in telemetry studies: The staggered entry design. The Journal of Wildlife Management 53: 7-15.
Rudas, A. & A. Prieto. 2004. Flórula del Parque Nacional Natural Amacayacu. Missouri Botanical Garden Press. San Luis, Missouri.
Sallabanks, R. & S. P. Courtney. 1992. Frugivory, seed predation, and insect-vertebrate interactions. Annual Review of Entomology 37: 377-400.
Schultes, R. E. 1977. Promising structural fiber palms of the Colombian Amazon. Principes 21: 72-82.
Smythe, N. 1989. Seed removal in the palm Astrocaryum standleyanum: Evidence for dependence upon its seed dispersers. Biotropica 21: 50-56.
SPSS v.14. 2005. SPSS. Chicago.
Silvius, K. M. & J. M. Fragoso. 2003. Redrumped agouti (Dasyprocta leporina) home range use in an Amazonian forest: implications for the aggregated distribution of forest trees. Biotropica 35: 74-83.
Stevenson, P.R., M.J. Quiñones & M.C. Castellanos. 2000a. Guía de frutos de los bosques del río Duda, Macarena, Colombia. Asociación para la Defensa de La Macarena - IUCN. Bogotá. 650 p.
Stevenson, P. R., M. J. Quiñones & J. A. Ahumada. 2000b. Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica 32: 533-544.
Terborgh, J. G. Núñez-Iturri, N.C.A. Pitman, F.H. Cornejo-Valverde, P. Álvarez, V. Swamy, E. G. Pringle & C. E. T. Paine. 2008. Tree recruitment in an empty forest. Ecology 89: 1757-1768
UAESPNN. 1999. Diagnóstico y estrategias de conservación de las poblaciones de fauna silvestre con mayor presión de caza en el sector sur del Parque Nacional Natural Amacayacu. Informe final. Parque Nacional Natural Amacayacu, U.A.O.y.P. (ed.). Ministerio de Ambiente, Vivienda y Desarrollo Territorial.
Vormisto, J. 2002. Making and marketing chambira hammocks and bags in the village of Brillo Nuevo, Northeastern Peru. Economic Botany 56: 27-40.
Xiao, Z., P. A. Jansen & Z. Zhang. 2006a. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. Forest Ecology and Management 223: 18-23.
Xiao, Z., Y. Wang, M. Harris & Z. Zhang. 2006b. Spatial and temporal variation of seed predation and removal of sympatric large-seeded species in relation to innate seed traits in a subtropical forest, Southwest China. Forest Ecology and Management 222: 46-54.
Licencia
Colombia Forestal conserva los derechos patrimoniales (copyright) de las obras publicadas, y favorece y permite la reutilización de las mismas bajo la licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional por lo cual se pueden copiar, usar, difundir, transmitir y exponer públicamente, siempre que:
Se reconozcan los créditos de la obra de la manera especificada por el autor o el licenciante (pero no de una manera que sugiera que tiene su apoyo o que apoyan el uso que hace de su obra).