DOI:
https://doi.org/10.14483/23448350.20875Publicado:
05/15/2023Número:
Vol. 47 Núm. 2 (2023): Mayo-Agosto 2023Sección:
ArtículosAqueous Pretreatment Effect to Improve Au, Ag, and Pt Recovery from Spent Automotive Catalysts
Efecto de pretratamiento acuoso para mejorar la recuperación de Au, Ag y Pt a partir de catalizadores automotrices gastados
Palabras clave:
leaching, precious metals, waste automotive catalysts (en).Palabras clave:
Lixiviación, metales preciosos, catalizadoes automotrices de desecho (es).Descargas
Referencias
Avery, H. E. (1974). Basic reaction kinetics and mechanisms. Macmillan Publishers. DOI: https://doi.org/10.1007/978-1-349-15520-0
Borda, J., González, C., Torres, R. (2022a). Aqueous recovery of zinc and lead from coal fly ashes of a Colombian thermoelectric plant. Ingeniería e Investigación, 43(1), e95364. https://doi.org/10.15446/ing.investig.95364 DOI: https://doi.org/10.15446/ing.investig.95364
Borda, J., Torres, R. (2021). Comparative study of selective zinc leaching from EAFD using carboxylic agents. Revista Mexicana de Ingeniería Química, 20(1), 389-398. https://doi.org/10.24275/rmiq/IA2022 DOI: https://doi.org/10.24275/rmiq/IA2022
Borda, J., Torres, R. (2022b). Recycling of zinc and lead from electric arc furnace dust by selective leaching with EDTA. Canadian Metallurgical Quarterly, 61(4), 464-474. https://doi.org/10.1080/00084433.2022.2046902 DOI: https://doi.org/10.1080/00084433.2022.2046902
Borda, J., Torres, R. (2022c). Prospects for thiourea as a leaching agent in Colombian gold small-scale mining: A comprehensive review. Journal of Sustainable Mining, 21(4), 298-308. https://doi.org/10.46873/2300-3960.1364 DOI: https://doi.org/10.46873/2300-3960.1364
Benson, M., Bennett, C. R., Harry, J. E., Patel, M. K., Cross, M. (2000). The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resources, Conservation, and Recycling, 31(1), 1-7. https://doi.org/10.1016/s0921-3449(00)00062-8 DOI: https://doi.org/10.1016/S0921-3449(00)00062-8
Chauhan, G., Ram, P., Pant, K. K., Nigam, K. D. P. (2018). Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment : Challenges and opportunities – A review. Journal of Environmental Chemical Engineering, 6(1), 1288-1304. https://doi.org/10.1016/j.jece.2018.01.032 DOI: https://doi.org/10.1016/j.jece.2018.01.032
Eskina, V. V., Dalnova, O. A., Filatova, D. G., Baranovskaya, V. B., Karpov, Y. A. (2020). Direct precise determination of Pd, Pt and Rh in spent automobile catalysts solution by high-resolution continuum source graphite furnace atomic absorption spectrometry. Spectrochimica Acta Part B, 165, e105784. https://doi.org/10.1016/j.sab.2020.105784 DOI: https://doi.org/10.1016/j.sab.2020.105784
Fajar, A. T. N., Hanada, T., Goto, M. (2021). Recovery of platinum group metals from a spent automotive catalyst using polymer inclusion membranes containing an ionic liquid carrier. Journal of Membrane Science, 629, e119296. https://doi.org/10.1016/j.memsci.2021.119296 DOI: https://doi.org/10.1016/j.memsci.2021.119296
Hammadi, M. Q., Abid, K. N. (2017). Recovery of platinum and palladium from scrap automotive catalytic converters. Al-Khwarizmi Engineering Journal, 13(3), 131–141. https://doi.org/10.22153/kej.2017.04.002 DOI: https://doi.org/10.22153/kej.2017.04.002
Hussaini, S., Kursunoglu, S., Top, S., Ichlas, Z. T., Kaya, M. (2021). Testing of 17-different leaching agents for the recovery of zinc from a carbonate-type Pb-Zn ore flotation tailing. Minerals Engineering, 168, e106935. https://doi.org/10.1016/j.mineng.2021.106935 DOI: https://doi.org/10.1016/j.mineng.2021.106935
Kaya, M. (2018). Current WEEE recycling solutions. In F. Vegliò & I. Birloaga (Eds.), Waste Electrical and Electronic Equipment Recycling (pp. 33-93). Elsevier. https://doi.org/10.1016/B978-0-08-102057-9.00003-2 DOI: https://doi.org/10.1016/B978-0-08-102057-9.00003-2
Moeller, C. (1995). Quimica Inorganica. Reverte.
NIST (2004). Critically selected stability constants of metal complexes. 46 NIST Standard Reference Database (Version 8.0). NIST.
Poisot-Díaz, M. E., González, I., Lapidus, G. T. (2008). Electrodeposition of a silver-gold alloy (DORÉ) from thiourea solutions in the presence of other metallic ion impurities. Hydrometallurgy, 93(1-2), 23-29. https://doi.org/10.1016/j.hydromet.2008.02.015 DOI: https://doi.org/10.1016/j.hydromet.2008.02.015
Puigdomenech, I. (2004). Make Equilibrium Diagrams Using Sophisticated Algorithms
(MEDUSA). Inorganic Chemistry, Royal Institute of Technology. https://sites.google.com/site/chemdiagr/
Saguru, C., Ndlovu, S., Moropeng, D. (2018). A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy, 182, 44-56. https://doi.org/10.1016/j.hydromet.2018.10.012 DOI: https://doi.org/10.1016/j.hydromet.2018.10.012
Torres, R., Lapidus, G. T. (2016a). Copper leaching from electronic waste for the improvement of gold recycling. Waste Management, 57, 131-139. https://doi.org/10.1016/j.wasman.2016.03.010 DOI: https://doi.org/10.1016/j.wasman.2016.03.010
Torres, R., Lapidus, G. T. (2016b). Platinum, palladium and gold leaching from magnetite ore, with concentrated chloride solutions and ozone. Hydrometallurgy, 166, 185-194. https://doi.org/10.1016/j.hydromet.2016.06.00 DOI: https://doi.org/10.1016/j.hydromet.2016.06.009
Torres, R., Lapidus, G. T. (2017). Closed circuit recovery of copper, lead and iron from electronic waste with citrate solutions. Waste Management, 60, 561-568. https://doi.org/10.1016/j.wasman.2016.12.001 DOI: https://doi.org/10.1016/j.wasman.2016.12.001
Torres, R., Lapidus, G. T. (2020). Base metal citrate pretreatment of complex ores to improve gold and silver leaching with thiourea. Hydrometallurgy, 197, e105461. https://doi.org/10.1016/j.hydromet.2020.105461 DOI: https://doi.org/10.1016/j.hydromet.2020.105461
Wiecka, Z., Rzelewska-Piekut, M., Regel-Rosocka, M. (2022). Recovery of platinum group metals from spent automotive converters by leaching with organic and inorganic acids and extraction with quaternary phosphonium salts. Separation and Purification Technology, 280, e119933. https://doi.org/10.1016/j.seppur.2021.119933 DOI: https://doi.org/10.1016/j.seppur.2021.119933
Wittstock, A., Biener, J., Bäumer, M. (2010). Nanoporous gold: A new material for catalytic and sensor applications. Physical Chemistry Chemical Physics, 12(40), 12919-12930. https://doi.org/10.1039/c0cp00757a DOI: https://doi.org/10.1039/c0cp00757a
Yajun, W., Xiaozheng, L. I. (2012). Health risk of platinum group elements from automobile catalysts. 2012 International Symposium on Safety Science and Technology, 45, 1004-1009. https://doi.org/10.1016/j.proeng.2012.08.273 DOI: https://doi.org/10.1016/j.proeng.2012.08.273
Yakoumis, I., Panou, M., Moschovi, A. M., Panias, D. (2021). Recovery of platinum group metals from spent automotive catalysts: A review. Cleaner Engineering and Technology, 3, e100112. https://doi.org/10.1016/j.clet.2021.100112 DOI: https://doi.org/10.1016/j.clet.2021.100112
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Recibido: de febrero de 2023; Aceptado: de mayo de 2023
Resumen
Los catalizadores automotrices representan una fuente secundaria de recuperación de metales preciosos. Usualmente estos elementos son obtenidos de minerales. Recientemente se han buscado métodos para recuperarlos a partir de materiales de desecho. La principal ruta consiste en tratamientos pirometalúrgicos a elevadas temperaturas con la generación de otros contaminantes y un elevadísimo consumo energético. Con la hidrometalurgia, se pueden desarrollar rutas que favorezcan la extracción acuosa de dichos elementos a temperatura ambiente. En un catalizador automotriz, hay distintos metales que pueden interferir en el proceso de recuperación. Este estudio evaluó citrato de sodio (Na3C6H5O7•H2O), ácido nítrico (HNO3) y ácido clorhídrico (HCl) como pretratamiento hidrometalúrgico para disolver Fe, Zn y Pb contenidos en un catalizador automotriz gastado y mejorar la extracción posterior de los metales preciosos contenidos en el mismo (Au, Ag y Pt). Posteriormente, se realizó la extracción acuosa de elementos preciosos con tiourea (SC(NH2)2), comparando el efecto de los reactivos de pretratamiento. Finalmente, se analizaron la viabilidad y el efecto de cada pretratamiento como ruta en la recuperación de metales preciosos, a fin de evitar procesos a altas temperaturas.
Palabras clave:
catalizadores automotrices de desecho, lixiviación, metales preciosos..Abstract
Automotive catalysts represent a secondary source for precious metals recovery. These elements are usually obtained from minerals. Recently, methods have been sought to recover them from waste materials. The main route consists of pyrometallurgical treatments at high temperatures with the generation of other pollutants and very high energy consumption. With hydrometallurgy, routes can be developed which favor the aqueous extraction of said elements at room temperature. In an automotive catalyst, there are different metals which can interfere in the recovery process. In this study, sodium citrate (Na3C6H5O7•H2O), nitric acid (HNO3), and hydrochloric acid (HCl) were evaluated as a hydrometallurgical pretreatment to dissolve Fe, Zn, and Pb contained in a spent automotive catalyst and to improve the subsequent extraction of the precious metals contained therein (Au, Ag, and Pt). Afterwards, the aqueous extraction of precious elements with thiourea (SC(NH2)2) was carried out, comparing the effect of the pretreatment reagents. Finally, the feasibility and effect of each pretreatment as a route for precious metals recovery were analyzed with the aim to avoid processes at high temperatures.
Keywords:
leaching, precious metals, spent automotive catalysts..Resumo
Catalisadores automotivos representam uma fonte secundária de recuperação de metais preciosos. Normalmente, esses elementos são obtidos a partir de minerais. Recentemente, têm-se procurado métodos para recuperá-los a partir de materiais residuais. A rota principal consiste em tratamentos pirometalúrgicos em altas temperaturas com geração de outros poluentes e consumo de energia muito alto. Com hidrometalurgia podem ser desenvolvidas rotas que favorecem a extração aquosa dos referidos elementos à temperatura ambiente. Em um catalisador automotivo existem diversos metais que podem interferir no processo de recuperação. Neste estudo, citrato de sódio (Na3C6H5O7•H2O), ácido nítrico (HNO3) e ácido clorídrico (HCl) foram avaliados respectivamente como pré-tratamento hidrometalúrgico para a dissolução de Fe, Zn e Pb contidos em um catalisador automotivo usado e para melhorar a extração posterior dos metais preciosos nele contidos (Au, Ag e PT). Posteriormente, foi realizada a extração aquosa de elementos preciosos com tioureia (SC(NH2)2), comparando o efeito do reagente de pré-tratamento. Por fim, analisou-se a viabilidade e o efeito de cada pré-tratamento como rota na recuperação de metais preciosos, a fim de evitar processos em altas temperaturas.
Palavras-chaves:
lixiviação, metais preciosos, resíduos de catalisadores automotivos..Introduction
In the automotive industry, precious metals, especially those of the platinum group, are used for manufacturing catalysts (Eskina et al., 2020). An automotive catalyst is also composed of various base metals, such as Fe, Al, Zn, and Ni, and potentially toxic metals such as Pb (Wiecka et al., 2022), which can interfere in the recovery of aqueous precious metals.
When catalysts are incorrectly disposed of, they add to tons of solid waste with a high metallic content, which does not often undergo adequate elimination, recovery, or recycling processes (Yajun and Xiaozheng, 2012).
These automotive components are an important secondary source of precious metals and complement extraction from natural mines, which, for environmental reasons, tends to be a more complex process.
Metals recovery from catalysts is carried out through pyrometallurgical (high temperatures, dry route) and hydrometallurgical (room temperature, aqueous route) processes.
Pyrometallurgical processes have been used mainly for recycling precious metals. However, they have only been shown to be suitable on a large scale (Saguru et al., 2018). Because they demand high energy consumption, they require several stages, such as calcination, pyrolysis, combustion, and smelting, among other thermal processes, at temperatures between 1500 and 1900 °C.
In addition, the gases produced need treatment before being released into the atmosphere (Chauhan et al., 2018), and fine dust represents not only a risk of metal and energy loss, but also health issues (Kaya, 2018).
Hydrometallurgical processes offer important advantages for precious metals recovery. They require less energy and lower investments. In addition, their particulate matter and gaseous emissions are considerably lower compared to those of pyrometallurgical processes (Chauhan et al., 2018). The hydrometallurgical route’s stages are leaching (chemical dissolution in an aqueous medium), concentration, and recovery of the metal or metal salts of interest (Saguru et al., 2018).
Metals recovery by aqueous treatment poses several challenges, such as the substitution of aggressive leaching agents or the optimization of processes to reduce the concentration of reagents and continue obtaining high extraction values. Both in pyrometallurgical and hydrometallurgical processes, pretreatments are carried out to improve the efficiency of precious metals dissolution, separating the base metals and removing impurities (Saguru et al., 2018).
In the selective leaching processes, primary leaching constitutes the pretreatment for concentrating metals of interest, reducing interferences and impurities in the subsequent leaching process. This study seeks to evaluate three reagents as a pretreatment for base metals removal, aiming to improve the subsequent leaching of precious metals contained in used automotive catalyst material (Figure 1A). The reagent selection was based on results reported in the literature regarding base metals removal from different types of industrial waste (Torres and Lapidus, 2017; Borda et al., 2022a). The results were compared to those obtained by leaching the catalyst via thiourea without pretreatment, in order to verify the pretreatments’ effectiveness (Figure 1B).
Figure 1: (A) Procedure of the pretreatment stage (removal of base metals) with HCl, HNO3, and Citrate, for the subsequent leaching of precious metals with thiourea. (B) Metals leaching using thiourea without pretreatment.
Methodology
For this research, a spent commercial automotive catalyst was used. The catalyst was dismantled with a saw, separating the metallic casing from the honeycomb-like ceramic structure rich in precious and base metals. The honeycomb-type ceramic structure was subjected to conventional mechanical preparation processes until samples of 105 µm were obtained.
Digestion was performed with aqua regia at 80 °C and 200 rpm for chemical characterization. The elemental composition was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Agilent 4210 MP-AES).
Chemical pretreatment and leaching solutions, calibration standards, and dilutions for quantification were prepared with deionized water. The chemical reagents used in the study were of analytical grade.
With the MEDUSA software and the HYDRA database (NIST, 2004; Puigdomenech, 2004), dominance or Pourbaix diagrams were constructed for the thermodynamic study of acid-metal systems.
Hydrometallurgical Pretreatment
In this study, sodium citrate (hereinafter Cit-), HCl, and HNO3 were evaluated as leaching agents for the dissolution of the selected base metals. The reagents’ initial concentration was 0.5 M. For the agent with the best base metal dissolution result, the concentration varied between 0.1 and 1.0 M.
The pH of the Cit- solution was adjusted to 2 with a diluted HNO3 solution. 0.1 M hydrogen peroxide (50% w/v) were used to start the process as an oxidant for the metal phases.
The leaching process involved a solid/liquid ratio of 20 g/L, agitation stirrers of 500 rpm with compact paddle-type agitators (COLE-PARMER Model 50006-03), and room temperature (18 °C). Initial aliquots were taken every hour for 6 h.
The solution potential was monitored using a saturated Ag/AgCl electrode (Oakton pH ORP 700 Benchtop Meter), and the numerical values were adjusted to the standard hydrogen electrode (SHE).
The precious metal leaching solution was prepared with 0.5 M thiourea by adjusting the pH to 2 with dilute sulfuric acid solution. The leaching was carried out with the residue of the pretreatment that gave the best results. This residue was conventionally filtered and washed with deionized water, maintaining the same S/L ratio as the pretreatment. In this leaching phase, 0.1 M hydrogen peroxide were also added. All leachates were analyzed by atomic absorption spectrophotometry (AAS, Perkin Elmer 3110).
Results
Chemical characterization
The results of the chemical characterization confirmed the presence of precious metals in the sample and base metals (understood as metallic elements other than precious or radioactive ones) typical of the metallic composition of automotive catalysts (Eskina et al., 2020).
Platinum is essential because its catalytic activity is very high, while gold is responsible for oxidizing hydrocarbons and CO at low temperatures (Wittstock et al., 2010).
Table 1 shows that the base metals with the highest content in the sample are Fe, Zn, and Pb, while Ni and As report low amounts. Therefore, the monitoring of base metals was carried out for the three elements with the highest content.
Table 1: Elemental chemical composition of study sample.

Thermodynamic Analysis
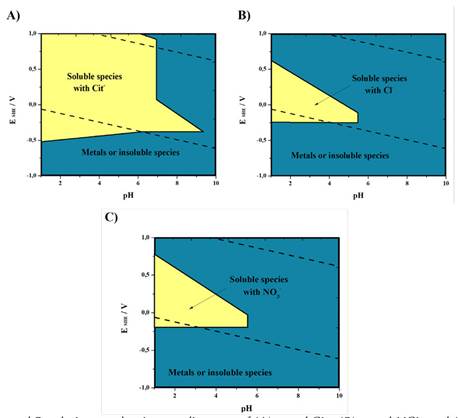
Through predominance diagrams, the ranges of thermodynamic conditions that favored the base metals leaching were determined (Figure 2). The reagents concentration used to construct the diagrams was 0.5 M, and that of the metals was 0.001 M. The yellow zone represents the pH and potential range where the evaluated base metals report at least one soluble species. The blue zone represents the range where at least one metal exhibits an insoluble species.
The metal-citrate system (Figure 2A) indicates a wide range of pH from 1 to approximately 6.8. The potential is not a limitation for the formation of soluble complexes. The experimentally measured potential was 530 mV. With the predominance diagram, it was determined that Cit- can be evaluated at a pH of 2 and yield soluble species.
The metal-HCl (Figure 2B) and metal- HNO3 (Figure 2C) systems show similar solubility zones. The range of potential for the production of soluble complexes is smaller and decreases with an increasing pH. According to the experiments, the HCl and HNO3 leaching solutions showed potentials of 530 and 600 mV, respectively.
Figure 2: Overlapped Pourbaix - predominance diagrams of (A) metal-Cit-, (B) metal-HCL, and (C) metal- HNO3 systems at 25 °C . Note: Designed with the MEDUSA software and NIST database (2004).
Pretreatment Agent Selection
The base metals extraction with Cit- (Figure 3) was 71 and 73% for Pb and Zn respectively. The results are consistent with the thermodynamic analysis of Figure 2A.
Each metal exhibited different dissolution kinetics. Considering that peroxide has an oxidizing effect on Fe, Zn, and Pb (Torres and Lapidus, 2016a), the oxidized Zn dissolution is better under acid conditions (Borda and Torres, 2021). Additionally, citrate ions have a greater affinity for some metal ions such as Pb(II) (Torres and Lapidus, 2017). Therefore, the selective behavior of the leaching is as expected. In the metal-HNO3 system, the extraction of Pb and Zn behaved similarly, reaching a maximum of 87% after 6 hours of leaching, coinciding with studies where HNO3 can dissolve both Zn and Pb in solution (Hussaini et al., 2021; Borda and Torres, 2022b). The oxidation of HNO3 in the base metals fosters a rapid dissolution. Figure 4 shows that, up to hour 3, the Pb and Zn species were more leachable than iron. After this time, the behavior of the extraction curve is asymptotic. This indicates that time is no longer an influential factor in metallic extraction.
Figure 3: Extraction of Fe, Zn, and Pb with Cit- 0.5 M and initial dosage of H2O2 0.1 M. Conditions: 18 °C, S/L 20g/L, 500 rpm, 6 h.
Figure 4: Extraction of Fe, Zn, and Pb with HNO3 0.5 M. Conditions: 18 °C, S/L 20g/L, 500 rpm, 6 h.
The pretreatment with HCl provided the highest base metals extraction percentages, obtaining 26, 94, and 95% of Fe, Zn, and Pb, respectively (Figure 5). In this system, there was also similarity in the behavior of the dissolution of Pb and Zn. The greatest base metals extraction was achieved during the first hour, after which extraction slowly increased as leaching continued.
Figure 5: Extraction of Fe, Zn, and Pb with HCl 0.5 M. Conditions: 18 °C, S/L 20g/L, 500 rpm, 6 h.
In this system, the dissolution of Fe was also limited by the potential. However, the experimental conditions were favorable for the formation of soluble species.
The three reagents used as pretreatment for the dissolution of base metals showed selectivity for Zn and Pb concerning Fe. Cit- offered extraction results similar to those obtained with HNO3. Nevertheless, HCl was the reagent that reached the highest extraction percentages.
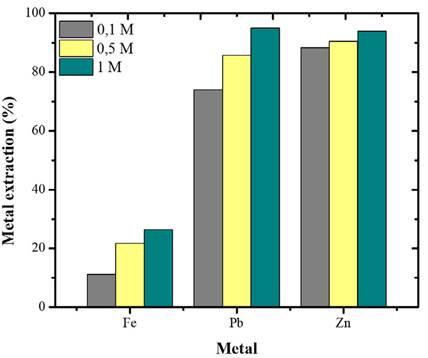
HCl Concentration Effect
The results show that, by decreasing the concentration to 0.1 M, the extraction is lower (Figure 6). The dissolution of the base metals under these conditions (0.1 M HCl) is similar to that achieved by Cit- at 0.5 M (Figure 3). Although Cit- shows a higher molar concentration in this comparison, Cit- solutions can replace 0.1 M HCl solutions for base metal leaching due to their lower toxicity potential.
Figure 6: Effect of HCl concentration on the leaching of base metals (Fe, Pb, Zn). Conditions: 18 °C, S/L 20g/L, 500 rpm, 6 h.
It was shown that increasing the molar concentration of HCl to 1.0 improves the extraction of the three metals, which is consistent with kinetic theory (Moeller, 1995; Avery, 1974). In Metal-Cl- systems, having a greater amount of reagent available in the solution implies a greater number of effective collisions between the molecules, allowing for a better formation of the products.
Despite the favorability of HCl at the highest molar concentration tested, its increase in the base metals extraction is not very significant. Therefore, the HCl concentration of the pretreatment tests was set to 0.5 M.
Thiourea Leaching of Precious Metals
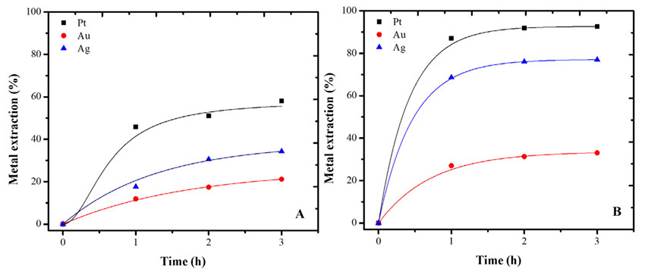
In order to compare the effect of pretreatment on precious metals leaching, a thiourea leaching test was performed on the pretreated sample, as well as another one on the raw sample (Figure 1A and B).
Thiourea can form complexes with silver and gold metal ions (Appendix), however, it is important to control the potential conditions of the reagent. Thus, the thiourea oxidation and the formation of its secondary products can be avoided, which alters its metallic dissolution capacity (Poisot-Díaz et al., 2008). The lower potentials are good for reducing thiourea consumption, but also, they decrease gold dissolution.
Platinum and silver were the precious metals by most favored the pretreatment (Figures 7A and 7B). The increase in aqueous extraction was 34 and 42% for Pt and Ag, respectively. The results showed that HCl pretreatment successfully removed lead, zinc, and some iron, thus freeing the precious metals for thiourea leaching.
Figure 7: Extraction of Pt, Au, and Ag using thiourea (A) without pretreatment and (B) with pretreatment. Conditions: thiourea pH 1.5, 0.5 M, initial activation with 0.1 M hydrogen peroxide.
Gold extraction also improves, but not in the same way (Figure 7B). After an hour and a half, the leaching time is no longer a significant factor in obtaining superior extractions. Generally, the kinetics of gold dissolution processes are controlled by the diffusion of reagents to the surface, and, therefore, it is linked to the concentrations of Fe (III), formamidine disulfide, and thiourea species (Borda and Torres, 2022c). A greater reduction of Fe in the pretreatment stage could ensure a better extraction of the precious metal.
The increase in extraction with pretreatment was 12%. Even so, it was shown that base metals removal benefits precious metals extraction from automotive catalysts. This contributes to the hydrometallurgical extraction of precious metals from different types of materials and minerals (Torres and Lapidus, 2016, 2020).
Further studies on metals recovery from solutions must be carried out to analyze the feasibility of recirculating them (Torres and Lapidus, 2017), thus turning the entire process into a sustainable alternative to the processes that are currently implemented (Benson et al., 2000; Yakoumis et al., 2021).
Conclusions
Spent automotive catalysts are a secondary means for precious metal recovery and can be integrated into circular economy processes. To improve precious metals extraction, it is convenient to carry out a pretreatment that helps to eliminate base metals and impurities (organic and volatile).
In this study, three reagents were evaluated as hydrometallurgical pretreatments, and it was found that 0.5 M sodium citrate at pH 2 with 0.1 M H2O2 can form soluble species of Fe, Zn, and Pb and provides aqueous extraction values similar to those obtained by the 0.1 M HCl solution. Meanwhile, with the HNO3 solution, high percentages of Zn and Pb extraction were achieved, and the leaching behavior for these two metals was the same. This inorganic reagent, like HCl and Cit-, exhibits selective leaching for Zn and Pb, achieving low Fe extraction results.
The most efficient reagent in the pretreatment was HCl, and, by increasing its molar concentration, the leaching of base metals was improved. Three hours are enough for the process, and the costs associated with the testing time are thus reduced.
It was shown that, although the pretreatment acted differently on the dissolution of Fe, Zn, and Pb, it was effective to improve the aqueous extraction of precious metals when leaching with thiourea. The most favorable conditions in this study were the following: hydrometallurgical pretreatment with 0.5 M HCl for 6 hours; leaching with 0.5 M thiourea at pH 1.5 with the activation of 0.1 M H2O2 for 3 hours. Under these conditions, final extractions of 92, 33, and 77% were achieved for Pt, Au, and Ag, respectively.
Acknowledgements
Acknowledgments
The authors are grateful for the support received from the VIE-SGI project to carry out this research, as well as from the Grupo Metalurgia No Ferrosa of Universidad Pedagógica y Tecnológica de Colombia.
References
Appendix
Licencia
Derechos de autor 2023 Adriana Vargas, Johana Borda, Robinson Torres

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
El (los) autor(es) al enviar su artículo a la Revista Científica certifica que su manuscrito no ha sido, ni será presentado ni publicado en ninguna otra revista científica.
Dentro de las políticas editoriales establecidas para la Revista Científica en ninguna etapa del proceso editorial se establecen costos, el envío de artículos, la edición, publicación y posterior descarga de los contenidos es de manera gratuita dado que la revista es una publicación académica sin ánimo de lucro.