DOI:
https://doi.org/10.14483/23448393.16525Published:
2020-12-19Issue:
Vol. 26 No. 1 (2021): January-AprilSection:
Petroleum EngineeringEvaluation of Extracts Obtained from Fruit Wastes Using Different Methods
Evaluación de extractos de residuos de frutas utilizando diferentes métodos
Keywords:
antioxidant activity, conventional extraction, fruit wastes, non-conventional extraction (en).Keywords:
capacidad antioxidante, extracción convencional, residuos de frutas, extracción no convencional (es).Downloads
References
L. V. Peñaranda, S. P. Montenegro, and P. A. Giraldo, “Aprovechamiento de residuos agroindustriales en Colombia”, Rev. Inv. Agr. Amb., vol. 8, no. 2, pp. 141–150, 2017. https://doi.org/10.22490/21456453.2040 DOI: https://doi.org/10.22490/21456453.2040
F. Bosco, A. Casale, G. Gribaudo, C. Mollea, and G. Malucelli, “Nucleic Acids from Agro-Industrial Wastes: A Green Recovery Meth-od for Fire Retardant Applications”, Ind. Crops Prod., vol. 108, pp. 208–218, 2017. https://doi.org/10.1016/j.indcrop.2017.06.035 DOI: https://doi.org/10.1016/j.indcrop.2017.06.035
J. Viganó and J. Martinez, “Trends for the Application of Passion Fruit Industrial By-Products: A Review on the Chemical Composition and Extraction Techniques of Phytochemicals”, Food Pub. Health, vol. 5, no. 5, pp. 164–173, 2015. https://doi.org/10.5923/j.fph.20150505.03 DOI: https://doi.org/10.5923/j.fph.20150505.03
N. A. Sagar, S. Pareek, S. Sharma, E. M. Yahia, and M. F. Lobo, “Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization”, Comp. Rev. F. Sci. F. Safe., vol. 17, pp. 512-531, 2018. https://doi.org/10.1111/1541-4337.12330 DOI: https://doi.org/10.1111/1541-4337.12330
M. K. Hrnčič, D. Cör, P. Kotnik, and Ž. Knez, “Extracts of White and Red Grape Skin and Rosehip Fruit: Phenolic Compounds and Their Antioxidative Activity”, Acta Ch. Slov., vol. 66, pp. 751-761, 2019. https://doi.org/10.17344/acsi.2019.5253 DOI: https://doi.org/10.17344/acsi.2019.5253
S. M. V. Palmeira, L. M. Gois, and L. D. Souza, “Extraction of Phenolic Compounds from Mango Peels”, Lat. Am. App. Res., vol. 42, pp. 77- 81, 2012.
M. Kaleem, A. Ahmad, T. Masud, and K. Raja, “Physico-Chemical Analysis and Optimization of Ultrasound Assisted Extraction of Phytochemicals from King’s Ruby Grapes”, The Journal of Animal & Plant Sciences, vol. 30, no. 1, pp. 205-211, 2020. https://doi.org/10.36899/JAPS.2020.1.0023 DOI: https://doi.org/10.36899/JAPS.2020.1.0023
V. Pezzini, F. Agostini, F. Smiderle, L. Touguinha, M. Salvador, and S. Moura, “Grape Juice By-Products Extracted by Ultrasound and Mi- crowaveassisted with Different Solvents: A Rich Chemical Composition”, Food Sci. Biotech., vol. 28, pp. 691-699, 2019. https://doi.org/10.1007/s10068-018-0531-x DOI: https://doi.org/10.1007/s10068-018-0531-x
G. Aguilar-Hernández, M. de L. García-Magaña, M. de Á. Vivar-Vera, S. G. Sáyago-Ayerdi, J. A. Sánchez-Burgos, J. Morales-Castro, L. M. Anaya-Esparza, and E. Montalvo-González, “Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Annona muricate By-Products and Pulp”, Molecules, vol. 904, no. 24, pp. 1-15, 2019. https://doi.org/10.3390/molecules24050904 DOI: https://doi.org/10.3390/molecules24050904
D. Grupta, “Methods for Determination of Antioxidant Capacity: A Review”, Int. J. Pharma. Sci. Res., vol. 6, no. 2, pp- 546-566, 2015. https://doi.org/10.13040/IJPSR.0975-8232.6(2).546-66 DOI: https://doi.org/10.13040/IJPSR.0975-8232.6(2).546-66
J. C. Camarena-Tello, H. E. Martínez-Flores, M. G. Garnica-Romo, J. S. Padilla-Ramírez, A. Saavedra-Molina, O. Alvarez-Cortes, M. C. Bar- tolomé-Camacho, and J. O. Rodiles-López, “Scavenging Abilities with Leaf Extracts from Two Varieties of Psidium Guajava L.”, Antioxidants, vol. 7, no. 34, pp. 2-12, 2018. https://doi.org/10.3390/antiox7030034 DOI: https://doi.org/10.3390/antiox7030034
A. Romulo, “The Principle of Some In vitro Antioxidant Activity Methods: Review”, IOP Conf. S.: Earth Env. Sci., vol. 426, pp. 1-7, 2020. https://doi.org/10.1088/1755-1315/426/1/012177 DOI: https://doi.org/10.1088/1755-1315/426/1/012177
A. S. Caballero, Evaluation of the Influence of Ultrasound and Supercritical Fluids in Processes of Polyphenolic Compounds Extraction from Agroindustrial Wastes, M.Sc dissertation, Chemical Engineering Department, Nat. U. Col., Manizales, Caldas, 2017.
Y. Poodi, M. Bimakr, A. Ganjloo, and S. Zarringhalami, “Intensification of Bioactive Compounds Extraction from Feijoa (Feijoa Sellowiana Berg.) Leaves Using Ultrasonic Waves”, Food Bioprod. Proc., vol. 108, pp. 37-50, 2018. https://doi.org/10.1016/j.fbp.2017.12.004 DOI: https://doi.org/10.1016/j.fbp.2017.12.004
O. R. Alara, N. H. Abdurahman, C. I. Ukaegbu, and N. A. Kabbashi, “Extraction and Characterization of Bioactive Compounds in Vernonia Amygdalina Leaf Ethanolic Extract Comparing Soxhlet and Microwave-Assisted Extraction Techniques”, J. Taibah U. Sci., vol. 13, no. 1, pp. 414-422, 2019. https://doi.org/10.1080/16583655.2019.1582460 DOI: https://doi.org/10.1080/16583655.2019.1582460
C. Da Porto, E. Porretto, and D. Decorti, “Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis Vinifera L.) Seeds”, Ultras. Sonoch., vol. 20, no. 4, pp. 1076–1080, 2013. https://doi.org/10.1016/j.ultsonch.2012.12.002 DOI: https://doi.org/10.1016/j.ultsonch.2012.12.002
I. F. F. Benzie and M. Devaki, “The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications”, in Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak R., Ca¸panoglu E. and Shahidi F., New Jersey, United States: John Wiley & Sons Ltd, pp. 77-106, 2017. https://doi.org/10.1002/9781119135388 DOI: https://doi.org/10.1002/9781119135388.ch5
H. S. Arruda, G. A. Pereira, D. R. de Morais, M. N. Eberlin, and G. M. Pastore, “Determination of Free, Esterified, Glycosylated and Insoluble-Boundphenolics Composition in the Edible Part of Araticum Fruit (Annona Crassiflora Mart.) and its By-Products by HPLC-ESI-MS/MS”, Food Ch., vol. 245, pp. 738–749, 2018. https://doi.org/10.1016/j.foodchem.2017.11.120 DOI: https://doi.org/10.1016/j.foodchem.2017.11.120
V. J. Cheng, A. Bekhit, M. McConnell, S. Mros, and J. Zhao, “Effect of Extraction Solvent, Waste Fraction and Grape Variety on the Antimicrobial and Antioxidant Activities of Extracts fromWine Residue from Cool Climate”, Food Ch., vol. 134, no. 1, pp. 474–482, 2012. https://doi.org/10.1016/j.foodchem.2012.02.103 DOI: https://doi.org/10.1016/j.foodchem.2012.02.103
H.H. Orak, I. S. Bahrisefit, and T. Sabudak, “Antioxidant Activity of Extracts of Soursop (Annona Muricata L.) Leaves, Fruit Pulps, Peels, and Seeds”, Pol. J. Food Nut. Sci., vol. 69, no. 4, pp. 359-366, 2019. https://doi.org/10.31883/pjfns/112654 DOI: https://doi.org/10.31883/pjfns/112654
M. E. A. O. Souza, N. Mezzomo, L. C. Correa, M. S. Lima, L. C. Azevêdo and S. R. S. Ferreira, “Recovery of Antioxidant Compounds from Mango Peel By Green Extraction Processes”, International Food Research Journal, vol. 26, no. 6, pp. 1845-1859, 2019.
O. M. Terrett and P. Dupree, “Covalent Interactions Between Lignin and Hemicelluloses in Plant Secondary Cell Walls”, Curr. Op. Biotech., vol. 56, pp. 97-104, 2019. https://doi.org/10.1016/j.copbio.2018.10.010 DOI: https://doi.org/10.1016/j.copbio.2018.10.010
K. S. Ojha, R. Aznar, C. O’Donnell, and B. K. Tiwari, “Ultrasound Technology for the Extraction of Biologically Active Molecules from Plant, Animal and Marine Sources”, Tr. A. Ch., vol. 122, pp. 1-10, 2020. https://doi.org/10.1016/j.trac.2019.115663 DOI: https://doi.org/10.1016/j.trac.2019.115663
A. Cvetanović, “Extractions without organic solvents: advantages and disadvantages”, Ch. Africa, vol. 2, pp. 343-349, 2019. https://doi.org/10.1007/s42250-019-00070-1 DOI: https://doi.org/10.1007/s42250-019-00070-1
T. W. Caldas, K. E.L. Mazza, A. S.C. Teles, G. N. Mattos, A. I. S. Brígida, C. A. Conte-Junior, R. G. Borguini, R. L.O. Godoy, L. M.C. Cabral, and R. V. Tonon, “Phenolic Compounds Recovery from Grape Skin Using Conventional and Nonconventional Extraction Methods”, Ind. Crops Prod., vol. 111, pp. 86-91, 2018. https://doi.org/10.1016/j.indcrop.2017.10.012 DOI: https://doi.org/10.1016/j.indcrop.2017.10.012
C. B. T. Pal and G. C. Jadeja, “Microwave-Assisted Extraction for Recovery of Polyphenolic Antioxidants from Ripe Mango (Mangifera Indica L.) Peel Using Lactic Acid/Sodium Acetate Deep Eutectic Mixtures”, Food Sci. Tech. Int., vol. 26, no. 1, pp. 78-92, 2019. https://doi.org/10.1177/1082013219870010 DOI: https://doi.org/10.1177/1082013219870010
M. H. Zeinab, A. S. Osheba, M. F. Khallaf, and A. A. Abdel, “Assessment of Grape Seeds as a Source of Antioxidant Compounds”, Arabs U. J. Agri. Sci., vol. 27, no. 1, pp. 501-509, 2019. https://doi.org/10.21608/AJS.2019.43659 DOI: https://doi.org/10.21608/ajs.2019.43659
Y. Li, J. Yao, C. Han, J. Yang, M. T Chaudhry, S. Wang, H. Liu, and Y. Yin, “Quercetin, Inflammation and Immunity”, Nutrients, vol. 167, no. 8, pp. 1-14, 2016. https://doi.org/10.3390/nu8030167 DOI: https://doi.org/10.3390/nu8030167
R. V. Patel, B. M. Mistry, S. K. Shinde, R. Syed, V. Singh, and H. S. Shin, “Therapeutic Potential of Quercetin as a Cardiovascular Agent”. Euro. J. Med. Ch., vol. 115, pp. 889-904, 2018. https://doi.org/10.1016/j.ejmech.2018.06.053 DOI: https://doi.org/10.1016/j.ejmech.2018.06.053
W. Z. Lee, S. K. Chang, H. E. Khoo, C. M. Sia, and H. S. Yim, “Influence of Different Extraction Conditions on Antioxidant Properties of Sour-sop Peel”, Acta Sci. Pol. Technol. Aliment., vol. 15, no. 4, pp. 419-428, 2016. https://doi.org/10.17306/J.AFS.2016.4.40 DOI: https://doi.org/10.17306/J.AFS.2016.4.40
B. Ayuda-Durán, S. González-Manzano, I. Gil-Sánchez, M. V. Moreno-Arribas, B. Bartolomé, M. Sanz- Buenhombre, A. Guadarrama, C. Santos-Buelga, and A. M. González-Paramás, “Antioxidant Characterization and Biological Effects of Grape Pomace Extracts Supplementation in Caenorhabditis elegans”, Foods, vol. 75, no. 8, pp. 1-14, 2019. https://doi.org/10.3390/foods8020075 DOI: https://doi.org/10.3390/foods8020075
A. Ruiz-Torralba, E. J. Guerra-Hernández, and B. García-Villanova, “Antioxidant Capacity, Polyphenol Content and Contribution to Dietary Intake of 52 Fruits Sold in Spain”, CyTA-J. Food, vol. 16, no. 1, pp. 1131-1138, 2018. https://doi.org/10.1080/19476337.2018.1517828 DOI: https://doi.org/10.1080/19476337.2018.1517828
N. Ćurko, K. Kelšin, V. Dragović-Uzelac, D. Valinger, M. Tomašević, and K. K. Ganić, “Microwave-Assisted Extraction of Different Groups of Phenolic Compounds from Grape Skin Pomaces: Modeling and Optimization”, Pol. J. Food Nut. Sci., vol. 69, no. 3, pp. 235-246, 2019. https://doi.org/10.31883/pjfns/109423 DOI: https://doi.org/10.31883/pjfns/109423
R. L. Prior, “Oxygen Radical Absorbance Capacity (ORAC): New Horizons in Relating Dietary Antioxidants/ Bioactives and Health Benefits”, J. Funct. Foods, vol. 18, pp. 797-810, 2015. https://doi.org/10.1016/j.jff.2014.12.018 DOI: https://doi.org/10.1016/j.jff.2014.12.018
S. Abhijit, S. J. Tripathi, V. Bhagya, S. Rao, M. V. Subramanyam, and S. A. Devi, “Antioxidant Action of Grape Seed Polyphenols and Aerobic Exercise in Improving Neuronal Number in the Hippocampus is Associated with Decrease in Lipid Peroxidation and Hydrogen Peroxide in Adult and Middle-Aged Rats”, Exp. Geront, vol. 101, pp. 101-112, 2018. https://doi.org/doi.org/10.1016/j.exger.2017.11.012 DOI: https://doi.org/10.1016/j.exger.2017.11.012
K. Yadav, R. Kumar, S. Mandal, P. Saha, and B. Mann, “Evaluation of Total Phenol Content and Antioxidant Properties of Encapsulated Grape Seed Extract in Yoghurt”, Int. J. Dairy Tech., vol. 71, no. 1, pp. 96-104, 2018. https://doi.org/10.1111/1471-0307.12464 DOI: https://doi.org/10.1111/1471-0307.12464
C. M. Ajila, K. A. Naidu, S. G. Bhat, and U. J. S. Prasada, “Bioactive Compounds and Antioxidant Potential of Mango Peel Extract”, Food Ch., vol. 105, pp. 982-988, 2007. https://doi.org/10.1016/j.foodchem.2007.04.052 DOI: https://doi.org/10.1016/j.foodchem.2007.04.052
Y. A. Vargas and L. I. Pérez, “Aprovechamiento de residuos agroindustriales en el mejoramiento de la calidad del ambiente”, Rev. Fac. Cien. Bás., vol. 14, no. 1, pp. 1-14, 2018. https://doi.org/10.18359/rfcb.3108 DOI: https://doi.org/10.18359/rfcb.3108
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Recibido: 16 de junio de 2020; Revisión recibida: 2 de septiembre de 2020; Aceptado: 21 de noviembre de 2020
Abstract
Context:
Currently, the increase in agroindustrial waste generation has encouraged the search for viable use alternatives. In this paper, four methods to obtain extracts from mango, soursop, and grape peels, as well as and grape seeds, are studied. Their efficiency is analyzed through extraction yields and antioxidant capacity characterization of the extracts.
Method:
The extraction was performed using solvent, Soxhlet, microwave-assisted, and ultrasound assisted extraction. The characterization of the extracts was made by total phenolic compounds and flavonoids quantification, as well as antioxidant capacity determination, using the DPPH, FRAP, and ORAC tests.
Results:
It was found that grape seed extracts obtained by different extraction methods, highlighting those obtained by microwave assisted extraction, present a high total content phenolic compounds (>321.381,41 ± 3.476,85 μg Gallic Acid/g) and flavonoids (>103.232,01 ± 4.638,19 μg Quercetin/g), in addition to high antioxidant activity, according to the results of the DPPH (<1,06 ± 0,01), FRAP (>152.280,08 ± 5.197,53 µg TROLOX/g), and ORAC (>124.566,81 ± 581,96 μg TROLOX/g) tests.
Conclusions:
The results presented in this study suggest that the extracts obtained from grape seeds, especially those obtained by means of microwave-assisted extraction, have a potential use in food and pharmaceutical industries, due to their high antioxidant capacity and their total phenolic compounds and flavonoids content.
Keywords:
antioxidant activity, conventional extraction, fruit wastes, non-conventional extraction..Resumen
Contexto:
En la actualidad, el aumento en la generación de residuos agroindustriales ha incentivado la búsqueda de alternativas viables de aprovechamiento. En este artículo se estudian cuatro métodos para la obtención de extractos a partir de cáscaras mango, guanábana y uva, y semillas de uva. Se analiza su eficiencia a través de los rendimientos de extracción y la caracterización de la capacidad antioxidante de los extractos.
Método:
La extracción se realizó mediante extracción con solvente, extracción Soxhlet, extracción asistida por microondas y extracción asistida por ultrasonido. La caracterización de los extractos se realizó mediante la cuantificación de compuestos fenólicos y flavonoides totales, así como la determinación de la capacidad antioxidante, utilizando las pruebas DPPH, FRAP y ORAC.
Resultados:
Se encontró que los extractos de semilla de uva obtenidos por diferentes métodos de extracción, destacando los obtenidos por extracción asistida por microondas, presentan alto contenido de compuestos fenólicos totales (>321.381,41 ± 3.476,85 µg Ácido Gálico/g) y flavonoides (>103.232,01 ± 4.638,19 µg Quercetina/g), además de una alta actividad antioxidante, según los resultados de las pruebas de DPPH (<1,06 ± 0,01), FRAP (>152.280,08 ± 5.197,53 µg TROLOX/g) y ORAC (>124.566,81 ± 581,96 µg TROLOX/g).
Conclusiones:
Los resultados presentados en este estudio sugieren que los extractos obtenidos de las semillas de uva, especialmente aquellos obtenidos mediante extracción asistida por microondas, tienen un uso potencial en la industria alimentaria y farmacéutica, debido a su alta capacidad antioxidante y su contenido de compuestos fenólicos totales y flavonoides.
Palabras clave:
capacidad antioxidante, extracción convencional, residuos de frutas, extracción no convencional..Introduction
Due to population growth and the need to improve living standards, the intensification of agricultural production and agroindustrial processes have caused an increase in waste volume [1]. Specifically, in the department of Caldas, a wide variety of agricultural products are generated, and some companies located in the department that process different fruits to produce fruit pulps, confectionery products, ice cream and dehydrated fruits, generate waste such as peels, seeds, stems, and stalks. This type of waste has a negative impact on the environment due to its high concentration of organic matter and its inappropriate final disposal [1]. Therefore, the interest in the use of agroindustrial waste has increased to mitigate environmental impacts, give added value to waste, and improve regional economies.
Mango, soursop, and grape peels, in addition to grape seeds, are some of the waste generated in the agroindustry of Caldas. These wastes have valuable compounds with antioxidant properties in their structure, which could be used as food ingredients, due to their benefits to human health [2]. The extraction of these compounds represents a viable and innovative alternative for the use of waste and for the development of regional industries, given that most of the waste of this type is used for the generation of energy or the production of concentrates for animals [1]. Valuable compounds could be extracted through conventional techniques, for example, through solvent, Soxhlet, and mechanical extraction [3]; and non-conventional techniques such as supercritical fluids extraction (SFE), ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE) [4]. Conventional extraction techniques have several disadvantages, since they consume too much time, are arduous, and have less selectivity and poor yield. They also use organic solvents that could be toxic. The basis of these techniques is basically the extraction power of the solvent and the heat applied or its combination [4].
Several authors have reported valuable compounds extraction using different techniques. Extraction yields of 60,00% were reported for grape peel using soxhlet extraction [5], and extraction yields between 29,00 and 34,00%in solvent extraction of mango peel compounds was obtained [6]. Some authors found yields around 2,00% in the MAE of compounds from grape wastes, [7], [8]. On the other hand, the UAE of compounds from the soursop peel has an approximate yield of 15,00% [9]. The results obtained previously in other studies are an indication of the potential of mango, soursop, and grape peels, as well as grape seeds, to be used to obtain valuable compounds with high added value.
Bioactive compound quantification and antioxidant capacity determination are carried out by spectrophotometric or colorimetric methods [10]. Some of the most used methods are the quantification of total phenolic compounds and flavonoids [11], ferric reducing antioxidant power (FRAP) determination [10], the 2,2 -diphenyl-1- picrylhydrazyl (DPPH) assay, the 2,2’-azinobis-(3-ethylben zthiazoline-6-sulphonic acid) (ABTS) assay, and oxygen radical absorption capacity (ORAC) determination [12]. The different antioxidant capacity assays are divided into hydrogen atom transfer (HAT) and single electron transfer (ET) reaction-based assays [10].
The aim of this article is to obtain extracts from four fruit wastes (mango peel, soursop peel, grape peel, and grape seed) using solvent extraction, Soxhlet extraction, microwave-assisted extraction, and ultrasound-assisted extraction. Also, the antioxidant capacity of the extracts was evaluated by determining total phenolic compounds and flavonoids, as well as performing DPPH, FRAP, and ORAC tests.
Methodology
Waste obtaining and pretreatment
Mango peel (MP), soursop peel (SP), grape peel (GP), and grape seeds (GS) were selected considering their antioxidant capacity, which was previously reported in the literature. The wastes were obtained from the industry in Manizales, Colombia. Fruit wastes were classified and sanitized for subsequent dehydration. These were cut manually to reduce size and dried at 45 °C in a Terrigeno brand muffle until constant weight. Once dry, the wastes were ground in a disk mill until a particle size of 1 mm or less was reached.
Solvent extraction (SOL)
The dried wastes were placed in a container with ethanol 60% in a 1:20 solid-liquid ratio. The procedure was carried out for 8 hours at a temperature of 25 °C and with stirring of 65 rpm [13].
Subsequently, the liquid fraction was separated from the solid fraction by vacuum filtration and subjected to rotaevaporation at 40 °C and 180 mbar in order to recover the solvent and concentrate the obtained extracts.
Soxhlet extraction (SOX)
The dried sample of the wastes was deposited in filter paper cartridges and placed on the Soxhlet equipment. The extraction was carried out for 8 hours, using ethanol at 60% with a 1:25 solid-liquid ratio [14]. The liquid fraction obtained was subjected to rotaevaporation at 40 °C and 180 mbar to recover the solvent and concentrate the extracts obtained.
Microwave-assisted extraction (MAE)
The dried wastes were deposited in a container with ethanol at 60% in a 1:10 solid-liquid ratio. For this procedure, a 22 factorial experimental design was used, in which the power and extraction time were selected as factors. Both factors had two levels, 700 W and 420 W for power and 1 and 3 min for time [15]. Subsequently, the liquid fraction was separated from the solid fraction by vacuum filtration and subjected to rotaevaporation at 40 °C and 180 mbar to recover the solvent and concentrate the obtained extracts.
Ultrasound-assisted extraction (UAE)
Wastes were deposited in a container with ethanol at 60% in a 1:20 solid-liquid ratio. The procedure was carried out for 1 hour at a constant temperature of 50 °C and a power of 50 W [13]. Subsequently, the liquid fraction was separated from the solid fraction by vacuum filtration and subjected to rotaevaporation at 40 °C and 180 mbar to recover the solvent and concentrate the obtained extracts.
Calculation of extraction yield
The extracts were weighed, and the crude extraction yield (EY) was calculated according to Eq. (1):

where ME is the extract mass (g) and MW is the waste mass (g).
Extract characterization
The characterization of the obtained extracts was carried out by quantifying the total phenolic compounds and flavonoids, as well as determining the antioxidant capacity with the DPPH, FRAP, and ORAC methods.
Total phenolic compound determination
The total phenolic compounds were determined following the methodology described in [16]. During the analysis, 60 μL of the sample were added to an assay tube that contained 4,75 mL of distilled water. 300 μL of FolinCiocalteu 1 N were added, homogenized, and allowed to react for 8 minutes. After this time elapsed, 900 μL of Na2CO3 20% were added and allowed to react for 2 hours. The absorbance was measured at 765 nm. A pattern of gallic acid (GA) was made, and distilled water was the photometric target. To this effect, the testing Spectroquant® Prove 300 spectrophotometer was utilized. The result was expressed as milligram equivalents of GA per gram of sample on a dry basis.
Flavonoid determination
Flavonoids were determined according to the methodology described in [11], with some modifications. For this test, 20 μL of the sample were taken, and 115 μL of water and 7,5 μL of NaNO2 5% were added, homogenized, and allowed to react for 5 minutes. Then, 30 μL of AlCl3 2,5% were added, homogenized, and allowed to react for 6 minutes. Finally, 50 μL of NaOH 1M and 50 μL of water were added, homogenized, and the absorbance was measured at 500 nm 5 minutes later. A pattern of quercetin was made, and the photometric blank was distilled water. This test was performed with a Multiskan GO UV/Vis Thermo Scientific microplate spectrophotometer. The result of this test was expressed in equivalent micrograms of quercetin per gram of dry-based sample.
Antioxidant activity determination
To determine the antioxidant capacity, the methodology proposed by [14] was used. According to this methodology, 5 dilutions of the sample were made, 150 μL of the dilution were taken and added to an assay tube that contained 3 mL of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) 6x10-5 M. The solution was stirred using a vortex for 30 seconds. Subsequently, the sample was stored in a dark place for 1 hour. Finally, the absorbance was recorded at 515 nm. For this test, a Spectroquant® Prove 300 spectrophotometer was utilized. The inhibition percentage was determined using Eq. (2), and the dilutions with values between 20 and 80% were taken to construct a graph of inhibition against concentration. From this graph, the equation to calculate the IC50 was found, which refers to the concentration of sample necessary to obtain a 50% inhibition of free radicals with DPPH.

where Inh is Inhibition, AbsDln is the dilution absorbance, and AbsBT is the blank test absorbance.
Ferric reducing antioxidant potential determination (FRAP)
The ferric reducing antioxidant potential was determined following the methodology described in [17]. For FRAP measurement, the FRAP reagent was prepared, which consists of a mixture of acetate buffer 300 mM, TPTZ 10 mM, and FeCl3 20 mM in a 10:1:1 ratio. First, 150 μL of the FRAP reagent were taken and incubated for one minute at 37 °C. They were then homogenized, and absorbance was measured at 600 nm. Subsequently, 20 μL of the sample were added, homogenized, and allowed to react for 8 minutes. Finally, absorbance at 600 nm was again measured. TROLOX was the standard substance, and the photometric blank was distilled water. For this test, a Multiskan GO UV/Vis Thermo Scientific microplate spectrophotometer was utilized. The result was expressed as equivalent micrograms of TROLOX per gram of dry-based sample.
Oxygen radical absorbance capacity determination (ORAC)
Oxygen radical absorbance capacity was determined according to the methodology described in [18]. For ORAC determination, 20 μL of the sample were taken, and 120 μL of fluorescein 120 nM are added and incubated for 15 minutes at 37 °C in the absence of light. Subsequently, 60 μL of AAPH 40 mM were added, and the fluorescein intensity was read every minute for 2 hours using 538 and 485 nm emission and excitation filters. For this analysis, TROLOX was used as the reference substance. The photometric target and positive control of the test was a phosphate buffer. This test was performed with a Fluoroskan Ascent Thermo Scientific microplate fluorimeter. The result was expressed as equivalent micrograms of TROLOX per gram of dry-based sample.
Statistical analysis
All analyses conducted in this study were performed in triplicate, and the values reported are presented as average values, along with their standard deviations. For the statistical analysis, the t-test was performed to identify whether the results obtained by every extraction method were significantly different. Solvent extraction was taken as the base case, so that the results obtained for the wastes by means of different extraction methods were compared with the results obtained by solvent extraction. p<0,05 was defined for significantly different data, and p<0,01 for significantly very different data. Statistical analysis was performed using Microsoft Excel®.
Results and discussion
Solvent extraction
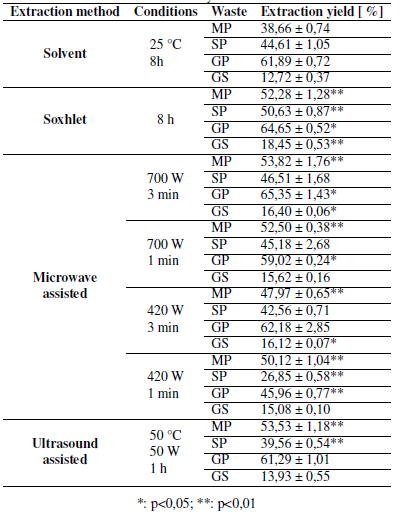
Table I presents the extraction yield obtained using solvent (SOL) and soxhlet (SOX) extraction. In SOL, the wastes with a highest content of extracts were grape peel (GP) and soursop peel (SP), with an extraction yield of 61,89 and 44,61 %, respectively. According to literature reports, the SOL yields for mango peel (MP) are between 29,00 and 34,00% [6], while the yield for grape seed (GS) is approximately 10,40% [19]. It was observed that the results obtained in this study (38,66% for MP and 12,72% for GS) are slightly higher compared to those reported in the literature. This is due to the extraction times used in previous studies [6], [19].
Table I: Extracts obtained by the different methods
Soxhlet extraction
In Table I, it is possible to observe that GP (64,65 %) and MP (52,28 %) present the highest content of extracts. Likewise, the yield through SOX extraction has significant differences in the case of GP and very significant differences for MP, SP, and GS, with respect to the extraction yield obtained through SOL extraction, which can be identified with asterisks presented in the Table and were obtained through statistical analysis.
In the literature, SOX extraction yield of GP is approximately 60,00% [5], a value close to that obtained by this study (64,65 %). However, in the case of SP, values from 0,23 to 16,50% are reported [20], showing a significant difference with the ones found in this work (50,63 %). This is possibly due to the use of other solvents, for example, methanol, hexane, or ethyl acetate, as well as the extraction temperature (room temperature) [20].
The differences obtained between the extraction yields with the SOX and SOL methods are mainly due to the fact that, in SOX extraction, waste is in contact with the pure solvent, while it is not recovered in SOL extraction, so it could become saturated [3]. Considering this, the performance of SOX extraction is expected to be higher.
Microwave-assisted extraction
Table I also presents the extraction yield obtained using microwave-assisted extraction (MAE). It is possible to observe that GP yields the highest content of extracts when powers of 700Wand 420 W and 3 minutes of extraction are used, with an EY of 65,35 and 62,18 %, respectively. According to previous studies, MAE has obtained yields of around 2,00% for grape wastes [7], [8], values much lower than those obtained in the present study, which vary between 15,08 and 65,35% for GS and GP. This information suggests that the selected operating conditions optimize the extraction process.
Table I also shows that, when the extraction time is set, if a higher power is used, higher extraction yields are obtained. On the other hand, if the extraction process power is set, increasing the time also increases the extracted compound yields, except for MAE, with a power of 420Wfor the MP case. Therefore, it can be concluded that the use of higher potential and longer extraction times positively influences the performance of the MAE process.
Similarly, it is observed that the extraction yield of MP by MAE (47,97-53,82 %) has very significant differences compared to SOL extraction (38,66 %). In the case of SP, only very significant differences are observed in MAE, with operating conditions of 420 W and 1 minute (26,85 for MAE and 44,61 for SOL). The extracts from GP show significant differences with respect to SOL extraction (61,89 %) when MAE is used at a power of 700 W and times of 1 and 3 minutes (65,35 and 59,02 %), while, if a power of 420 W is used for 1 minute (45,96 %), the differences are very significant. When GS is used to obtain extracts, it is observed that the yields obtained from these by MAE for 3 minutes (16,40 and 16,12 %) present significant differences compared to SOL extraction (12,72 %). Meanwhile, if the extraction is performed for 1 minute, no significant differences are reported.
Ultrasound-assisted extraction
Table I presents the extraction yield obtained using ultrasound assisted extraction (UAE). Wastes such as MP (53,53 %) and GP (61,29) yield a higher percentage of extracts compared to GS (13,93 %). According to a previous study [21], the yield of UAE for MP is 14,30 %, a value that differs considerably from the results obtained in this work (53,53 %). This is because the temperature used in the study was room temperature [21]. Similarly, a yield of 15,74% for SP was reported using operating conditions of 5 minutes and 25 °C [9]. This value is lower than the one obtained in this study (39,56 %). These data indicate that, at a higher temperature (50°C) and extraction time (1 h), it is possible to obtain better yields through UAE.
When the results obtained by the two non-conventional techniques are compared, slightly more favorable values are observed in the case of MAE at 700 W and 3 min, with 17% higher values for SP and GS. This may be due to the high lignin content present in the waste used for extraction, which has the function of giving resistance to the cell wall [22]. Considering the principle of acoustic cavitation that is applied in UAE, it could be suggested that the bubbles formed would not cause an easy rupture of the cell wall that allows a better diffusion of the solvent, and therefore a greater extraction yield [23].
Considering the consumption of time and energy of every extraction method, the time used in conventional extractions is significantly greater than the time required by non-conventional methods to obtain close extraction yields. This demonstrates that non-conventional extraction techniques represent a viable alternative to obtain valuable compounds, since operating times are noticeably shorter, which could be related to increased production. The energy consumption in non-conventional techniques is much lower, which translates into lower costs and, similarly, less environmental impact. On the other hand, it is important to highlight the use of ethanol as a solvent, as well as its possibility of recovery, which, in turn, translates into lower costs for the acquisition of raw materials, in addition to generating a low environmental impact by not disposing of organic solvents, characterized by negative impacts on the environment [24].
Extract characterization
Table II shows the results obtained for the total phenolic compounds (TPC) of the extracts, with values from 62.915,99 ± 889,91 μg GA/g for MP extracts to 700.936,84 ± 5.564,79 μg GA/g for GS extracts. In general, it is observed that the TPC contents of the extracts obtained by the different techniques for each of the wastes show significant differences with respect to the extracts obtained by solvent extraction, with 32 and 64% higher values for MP and GP extracts, respectively. On the other hand, when the extracts obtained by different methods were analyzed, it was found that there is no relationship between the extraction method and the TPC content of the extract. However, in the case of grape waste extracts, better results were found through MAE and SOX extraction.
Table II: Total phenolic compounds and flavonoids in the extracts

According to the results reported in the literature, the TPC values for GP extracts can vary from 5.000,00 to 48.600,00 μg GA/g with different extraction methods [25]. Previous studies also report TPC values between 3.512,00 and 5.617,00 μg GA/g for MP extracts [26]. When comparing data found in the literature with the results obtained in this study, it is observed that they differ in an order of magnitude (62.915,99 ± 889,91 to 147.193,63 ± 1.643,39 μg GA/g for MP extracts and 268.546,27 ± 9.779,74 to 613.037,87 ± 1.885,09 for GP extracts), which may be due to the concentration of the solvent, the time, and the extraction temperature, which are determining factors in extraction performance.
Table II also shows the results obtained from flavonoids for the extracts. Asterisks mark the significant (*) and very significant (**) differences in the flavonoid content of each extract obtained through the different methods with respect to solvent extraction. In general, GP and MP show significant differences, whereas for GS and SP, the differences between the results are not significant. On the other hand, according to the results presented in the literature, the GS extracts obtained by SOL extraction have a flavonoid content of 6.398,00 μg Quercetin/g [27], while the SP extracts obtained with SOX extraction present a flavonoid content of 36.100,00 μg Quercetin/g [20]. The differences between the results reported in the literature and those found in the present study (207.832,52 ± 13.996,46 μg Quercetin/g for GS extracts and 17.198,74 ± 780,13 μg Quercetin/g for SP extracts) are due to the different operating conditions and the use of solvents other than ethanol.
Likewise, it is observed that the higher flavonoid content corresponds to the extracts obtained from GS (103.232,01 ± 4.638,19 to 270.954,93 ± 10.582,09 μg Quercetin/g), followed by the extracts obtained from MP (62.674,99 ± 1.547,61 to 147.483,54 ± 4.269,63 μg Quercetin/g). A daily quercetin intake of approximately 50 mg has anti-inflammatory and antioxidant effects [28]. Therefore, MP and GS extracts, could supply of the recommended daily amount of quercetin. Consequently, these extracts could be used as food additives or dietary supplements [29].
The results obtained in the determination of the antioxidant capacity are presented in Table III. In general, it is observed that the antioxidant capacity (IC 50 , FRAP and, ORAC) of the extracts obtained by each of the methods presents significant differences with respect to the results obtained through SOL. In the case of the antioxidant activity (IC 50 results), the values could be 55 and 36% higher for MP and SP extracts, respectively, compared to the results obtained through solvent extraction. In the FRAP test, the results may differ in percentages of 37 and 62% for the extracts of MP and GS, compared to the values obtained for the extracts obtained with SOL. Likewise, differences of greater than 85% were found for the extracts of each waste, according to the results of the ORAC test. This can be seen in Table III, where * corresponds to a significant difference and ** is a very significant difference.
Table III: Antioxidant capacity of extracts
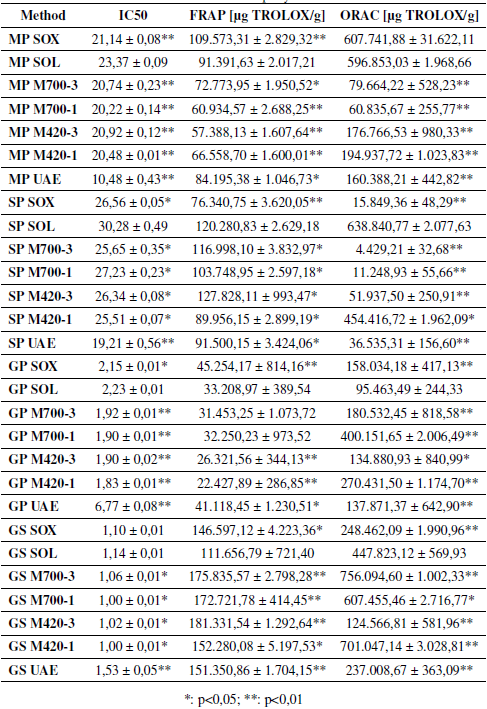
Table III shows that the extracts obtained from GS (1,00 ± 0,01 to 1,14 ± 0,01) and GP (1,83 ± 0,01 to 6,77 ± 0,08) have a greater inhibitory capacity expressed in terms of inhibitory concentration (IC 50 ), while the extracts obtained from SP (19,21 ± 0,56 to 30,28 ± 0,49) and MP (10,48 ± 0,43 to 23,37 ± 0,09) have the lowest inhibitory capacity. A literature study reports an IC 50 value of 1.180,00 for SP extract obtained through conventional extraction [30]. This value is higher than the one found in this work (30,28 ± 0,49), which is due to the extraction time of 1 hour and the use of pure ethanol as a solvent. On the other hand, an IC 50 value of 2,44 is reported for the GS extract, which does not differ greatly with the values obtained in this study (1,00 ± 0,01 to 1,14 ± 0,01).
In the case of the ferric reducing antioxidant potential (FRAP), it is observed that the extracts obtained from GS have the best results, highlighting those obtained by MAE (152.280,08 ± 5.197,53 to 181.331,54 ± 1.292,64 μg TROLOX/g). In previous studies, it has been found that the extracts of grape waste can reach FRAP values of 132.653,70 μg TROLOX/g [31], values close to those obtained in this study for GS (between 152.280,08 ± 5.197,53 and 181.331,54 ± 1.292,64 μg TROLOX/ g). On the other hand, it is observed that GS, GP, SP, and MP extracts present a greater FRAP value compared to raspberry and blackberry, which have a FRAP value of 9.828,89 and 9.999,09 μg TROLOX/g, respectively, and stand out for having a high antioxidant capacity [32].
Similarly, in this Table shows that the results obtained for oxygen radicals absorbance capacity (ORAC) range from 4.429,21 ± 32,68 μg TROLOX/g for SP extracts obtained by MAE at 700 W for 3 minutes, up to 756.094,60 ± 1.002,33 μg TROLOX/g for GS extract obtained by MAE at 700 W for 3 minutes. When comparing the results obtained with data reported in the literature for GP extracts, it is observed that ORAC values found in the pre- sent study are up to 12 times higher. In the case of MAE using methanol 60% as a solvent, an ORAC value of 31.982,06 μg TROLOX/ g [33] was reported, whereas for SOL, a value of 68.204,03 μg TROLOX/g was found [25]. This indicates that the operating conditions used in the extraction significantly affect the antioxidant capacity of the extracts.
In the daily diet, it is recommended to consume foods that provide an ORAC value greater than 2.502.900 μg of TROLOX [34]. According to this information, the extracts obtained from wastes can contribute an important percentage of these requirements, as is the case of the GS extracts obtained by microwave-assisted extraction at 700 W and 3 minutes (756.094,60 ± 1.002,33 μg of TROLOX), which can supply 30% of the antioxidants recommended for daily intake with 1 g of the extract. Therefore, these extracts could be a potential source of valuable compounds with the ability to inhibit lipid oxidation reactions [10].
According to the results, it is observed that GS extracts present the highest content of phenolic compounds (291.277,55 ± 1.5647,58 to 700.936,84 ± 5.564,79 μg GA/g) and a better antioxidant capacity, regardless of the way in which the extracts were obtained. Previous studies have reported the presence of compounds, for example, gallic acid, catechin, epicatechin, and proanthocyanidins in GS. These compounds contribute to the high antioxidant capacity of this waste [35], [36]. Considering the antioxidant capacity of GS extracts, the valuable compounds present in them would be expected to be good electron donors and can inhibit the Fe3/Ferricyanide complex, which is reflected in the high values of the FRAP test (111.656,79 ± 721,40 to 181.331,54 ± 1.292,64 μg TROLOX/g) [37]. The results obtained in the ORAC test (124.566,81 ± 581,96 to 756.094,60 ± 1.002,33 μg TROLOX/g) indicate that GS extracts contain compounds capable of trapping the peroxyl radical, which is relevant in the oxidation of lipids in food [10].
Considering the presence of antioxidant compounds in GS, this waste becomes a raw material of great interest in the food industry due to its benefits to human health [29]. The use of extracts obtained from fruit waste as food ingredients is an interesting alternative for obtaining compounds with a high antioxidant capacity, since it represents the possibility of valorization of this type of waste, which not only brings economic benefits for the industry, but also avoids inappropriate disposal, thus reducing environmental impact [38]. On the other hand, microwave-assisted extraction is a viable method for obtaining valuable compounds, since, in short periods of time and with low energy requirements and water consumption, it is possible to obtain high yields from the bioactive compounds with antioxidant capacity. However, it is necessary to analyze the composition of the extracts and carry out toxicity tests in order to define their possible applications and guarantee their safety.
Conclusions
The results obtained in this study show that the extraction yields of conventional and non-conventional methods turn out to be significantly different. Grape peel was the waste with the highest extraction yields, followed by mango peel, soursop peel, and grape seed. Obtaining grape and mango peel extracts proved to have higher yields when microwave-assisted extraction was used (700 W and 3 min), while the highest yield was obtained with Soxhlet extraction in the case of soursop peel and grape seed.
In contrast, grape seed extracts presented the highest antioxidant capacity, followed by mango peel, grape peel, and soursop peel. Grape peel, soursop peel, and grape seed extracts presented higher antioxidant capacity when microwave-assisted extraction was used. As for mango peel, the best results were for the extracts obtained through Soxhlet extraction.
These results indicate that there is no relationship between the extraction method, its efficiency, and the quality of the extracts obtained. This is due to the composition of the wastes, which may present compounds in their structure whose extraction is facilitated by a specific technique. However, microwave-assisted extraction presented very good results for each waste, and, considering the short extraction times, it is the technique with the highest efficiency.
The results of this study give rise to future research in which the composition of the extracts obtained is analyzed, especially those obtained from grape seeds by microwave-assisted extraction, in order to identify different metabolites and their concentration, and similarly, carry out toxicity tests to guarantee the safety of the extracts, so that they can be used as food ingredients with a potential specific benefit.
References
License
Copyright (c) 2020 Ciliana Florez Montes, Andrés Felipe Rojas González, Sneyder Rodríguez Barona

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
From the edition of the V23N3 of year 2018 forward, the Creative Commons License "Attribution-Non-Commercial - No Derivative Works " is changed to the following:
Attribution - Non-Commercial - Share the same: this license allows others to distribute, remix, retouch, and create from your work in a non-commercial way, as long as they give you credit and license their new creations under the same conditions.




2.jpg)